
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































> Solutions for In Vivo CAR-T Cell Therapy Development In vivo CAR T cell therapy is advancing into clinical trials! This groundbreaking approach aims to reprogram immune cells directly inside the body, offering a faster, more cost-effective, and potentially safer alternative to traditional ex vivo CAR T therapies.
Trends Pharmacol Sci. 2024;45(5):406-418.
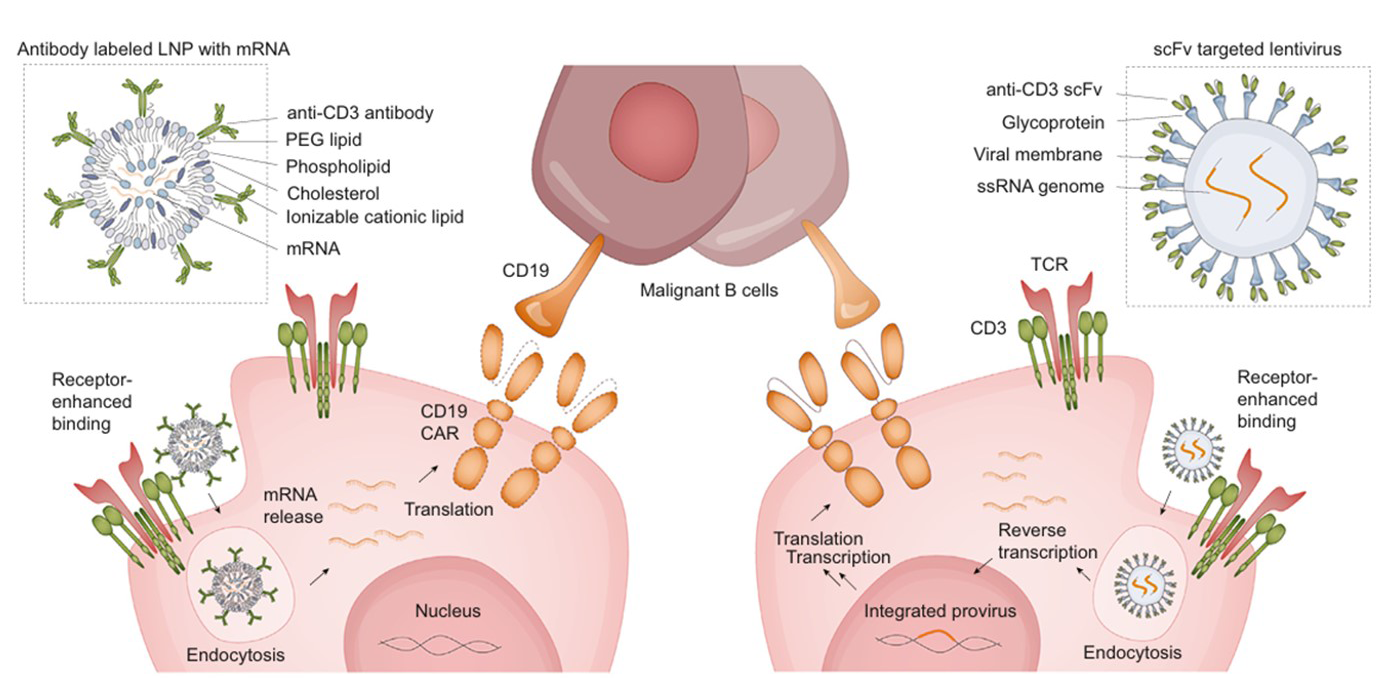
Workflow of In vivo CAR T Cell Therapy
A range of GMP-grade media, cytokines, antibodies, and other products to support in vitro activation and culture of T cells.
We offer a range of high-quality QC kits designed for comprehensive mRNA quality control, ensuring the integrity and reliability of mRNA in therapeutic and research applications. These kits are optimized for accurate assessment of mRNA purity, integrity, and functionality.
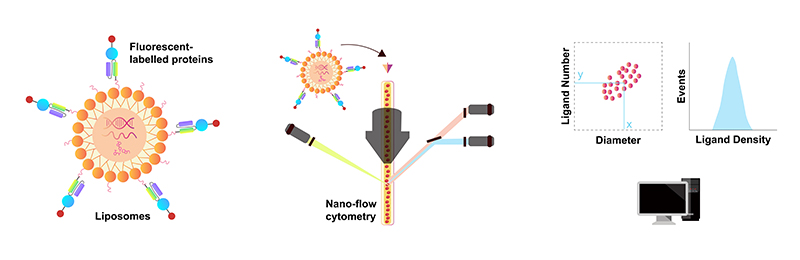
For in vivo CAR-T, receptor targeting of vector particles is crucial, as it can prevent CAR gene delivery into off-target cells, thus reducing toxicities. T cell biomarker (including CD8, CD3, CD5, CD7 and CD4) antibodies are usually conjugated to LNP or coated on Lentiviral for accurately targeting. Antibody density and activity is a critical quality attribute for the quality, safety, and efficacy of Ab-LNP. We've developed fluorescent-labeled proteins with stable F/P ratio and high activity for quantitative analysis of antibody density on Ab-LNPs.
 Alexa Fluor™ 488-Labeled Human CD8 alpha Protein, His Tag
Alexa Fluor™ 488-Labeled Human CD8 alpha Protein, His Tag
 Alexa Fluor™ 488-Labeled Human CD3 epsilon Protein, His Tag
Alexa Fluor™ 488-Labeled Human CD3 epsilon Protein, His Tag
 Alexa Fluor™ 488-Labeled Human CD5 Protein, His Tag Star Staining
Alexa Fluor™ 488-Labeled Human CD5 Protein, His Tag Star Staining
 Alexa Fluor™ 488-Labeled Human CD7 Protein, His Tag Star Staining
Alexa Fluor™ 488-Labeled Human CD7 Protein, His Tag Star Staining
 Alexa Fluor™ 488-Labeled Human CD4 Protein, His Tag Star Staining
Alexa Fluor™ 488-Labeled Human CD4 Protein, His Tag Star Staining

1e5 of Mouse Anti-CD7 antibody coupled beads (5.5 μm) were stained with different concentration of Alexa Fluor 647-Labeled Human CD7 Protein, His Tag (Cat. No. CD7-HA2H4) and negative control protein respectively, AF647 signal was used to evaluate the binding activity (QC tested).
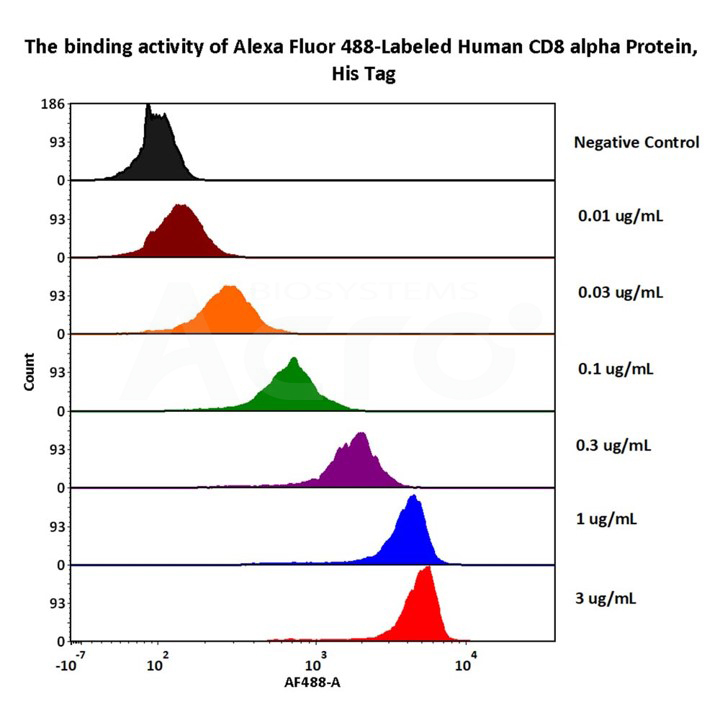
1e5 of Mouse Anti-CD8 antibody coupled beads (5.5 μm) were stained with different concentration of Alexa Fluor 488-Labeled Human CD8 alpha Protein, His Tag (Cat. No. CDA-HA2H6) and negative control protein respectively, AF488 signal was used to evaluate the binding activity (QC tested).
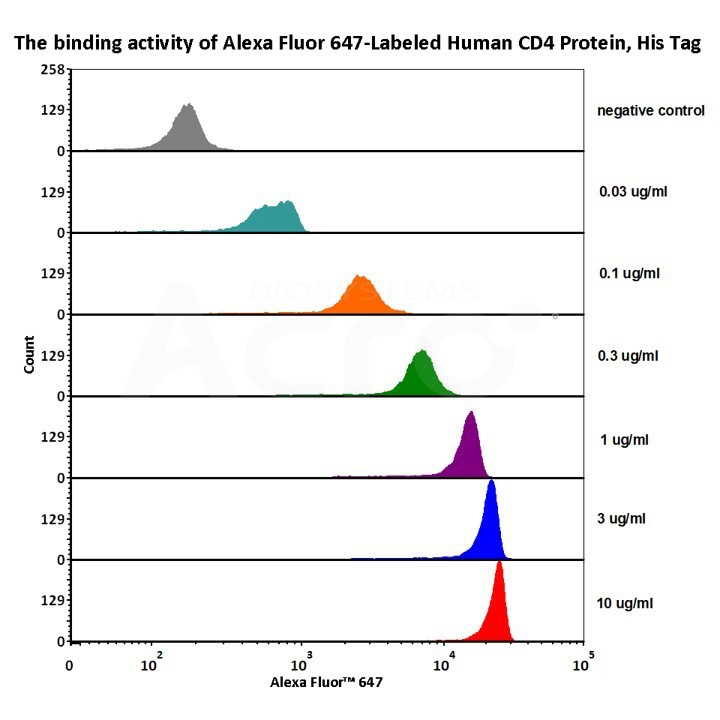
1e5 of Mouse Anti-CD4 antibody coupled beads (5.5 μm) were stained with different concentration of Alexa Fluor 647-Labeled Human CD4 Protein, His Tag (Cat. No. CD4-HA2H8) and negative control protein respectively, AF647 signal was used to evaluate the binding activity (QC tested).
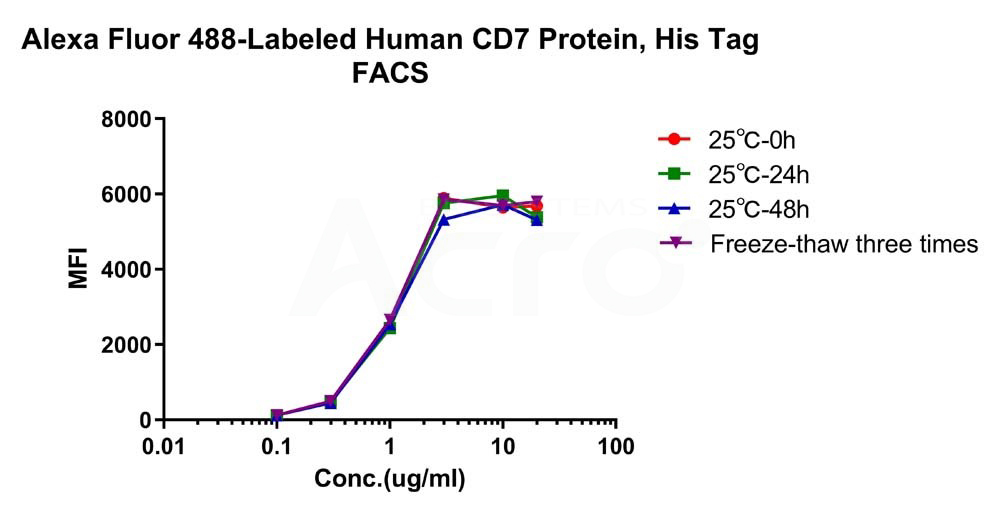
Alexa Fluor 488-Labeled Human CD7 Protein, His Tag (Cat. No. CD7-HA2H9) is stable at 25℃ for 48 hours, equivalent to store at -70℃ for 2 years and freezing and thawing 3 times without performance reduction.
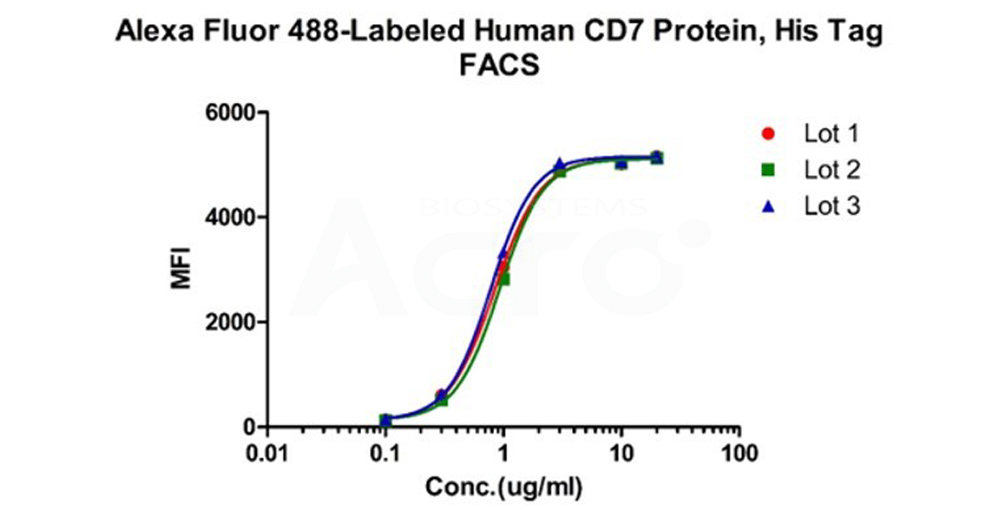
Binding activity of three different lots of Alexa Fluor 488-Labeled Human CD7 Protein, His Tag against Anti-CD7 CAR-293 cells was evaluated by flow cytometry. The result shows very high batch-to-batch consistency.
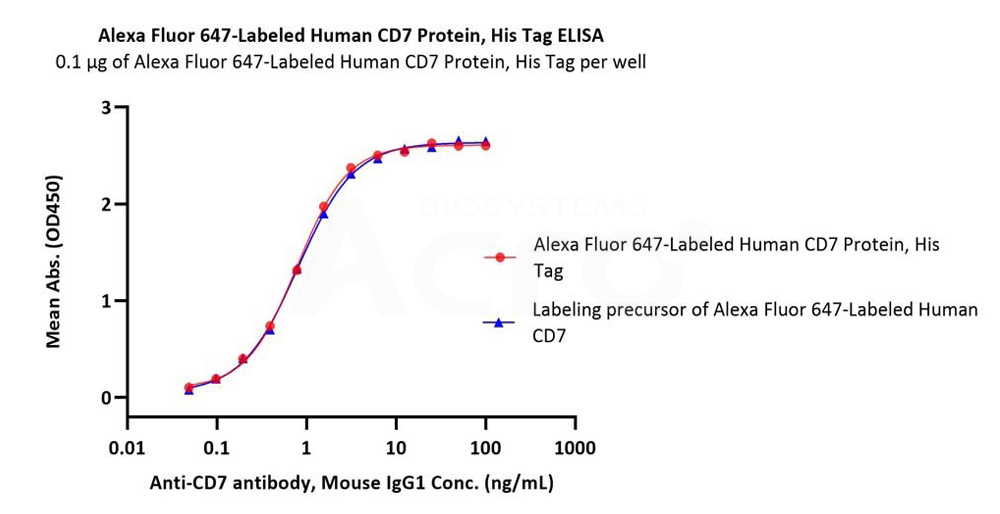
Immobilized Alexa Fluor 647-Labeled Human CD7 Protein, His Tag (Cat. No. CD7-HA2H4) at 1 μg/mL (100 μL/well) can bind Anti-CD7 antibody, Mouse IgG1 with a linear range of 0.05-3 ng/mL (Routinely tested). Labeling with fluorescent dyes did not affect their activity.
We have also developed an extensive collection of CAR target proteins, including fluorescently labeled target antigens and pre-biotinylated proteins, specifically designed for evaluating CAR expression.
Brochure—Solutions of Immune Cell Therapy Development
Brochure—Special Topic on Deep Interpretation of GMP Product Quality
Brochure—Star Staining - Fluorescent labeled Products
Brochure—resDetect™ Manufacturing Process Residue Detection Solutions-Residual Detection Kits
This web search service is supported by Google Inc.







