
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































> Insights > Mycoplasma Detection: A Critical Defense for Ensuring Biopharmaceutical Quality 
Mycoplasma is a type of bacteria known for being one of the smallest organisms capable of independent survival. There are over 190 species of mycoplasma, found in humans, animals, and plants. Unlike most bacteria, mycoplasma lacks a cell wall and survives by obtaining nutrients directly from host cells. Due to its tiny size (0.15-0.3 microns), it can easily evade filtration systems, making it hard to detect, even in high concentrations.
Mycoplasma contamination can cause multiple hazards to cell physiology, including inducing chromosomal abnormalities, interfering with DNA and RNA synthesis, altering membrane antigenicity, inhibiting cell proliferation and metabolism, reducing transfection rates, changing gene expression profiles, and causing cell death.
Various cell lines are widely used in producing therapeutic biologics like cytokines, vaccines, monoclonal antibodies, and immunomodulators. Advanced therapy medicinal products (ATMPs) based on cell and gene therapies have emerged as novel treatment options. However, the production process for autologous cell therapy is complex, involving multiple stages, each with potential mycoplasma contamination risks. Mycoplasma contamination poses significant hazards in cell therapy and biopharmaceutical industries. Once the cell matrix is contaminated, it may threaten patient safety and lead to product issues or recalls, causing severe economic losses. Therefore, routine mycoplasma testing is indispensable in biopharmaceutical production and R & D.
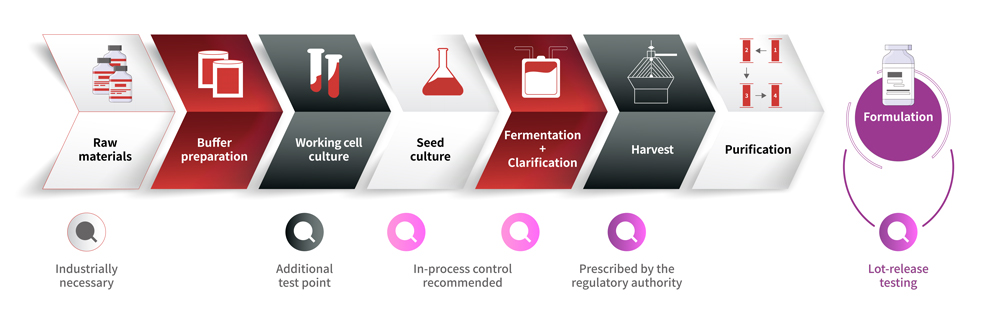
Monitoring Mycoplasma Contamination in Biopharmaceutical Production
Mycoplasma detection is essential throughout biologics production using cell lines. Common methods include culture-based assays, indicator cell-based assays, DNA staining assays, and PCR-based assays. Traditional culture-based and indicator cell-based assays are cumbersome, time-consuming(up to 28 days), and have low sensitivity. With the advancement of cell-based therapies and ATMPs, the requirements for mycoplasma detection timeliness and sensitivity are becoming increasingly stringent. PCR-based assays using nucleic acid amplification technology (NAT) have emerged as a rapid and reliable alternative. They offer quick setup and analysis, high sensitivity, specificity, reliability, and cost-effectiveness. Currently, major pharmacopeias like EP<2.6.7>, JP, and USP<63>have included NAT methods for mycoplasma detection.
To accelerate in-process and batch release testing, ACROBiosystems offers a comprehensive mycoplasma monitoring solution, including mycoplasma DNA sample preparation kit, and mycoplasma rapid detection kit. These kits ensure sensitive and reliable mycoplasma contamination detection in biologics, meeting regulatory guidelines of major pharmacopeias (10 CFU/ml).

• Wide Coverage: Detects over 250 mycoplasma and spiroplasma species, including all listed in EP, USP, and JP.
• High Specificity: Primers and probes target mycoplasma 16S rRNA, avoiding cross-reactivity with non-mycoplasma genera.
• High Sensitivity: Meets or exceeds regulatory standards of 10 CFU/mL.
• Strong Compatibility: Suitable for complex matrices like high cell density samples and 10% DMSO.
• User-friendly: Single-well assay design with included controls. Total assay time is only 2.5 - 3 hours.
• High Quality: Manufactured in GMP-like facilities, compliant with ISO 13485 standards, and validated according to relevant guidelines.
• Comparable Results: Assay results are comparable to culture-based methods.
• Wide Coverage: Capable of detecting over 250 mycoplasma species and spiroplasma.
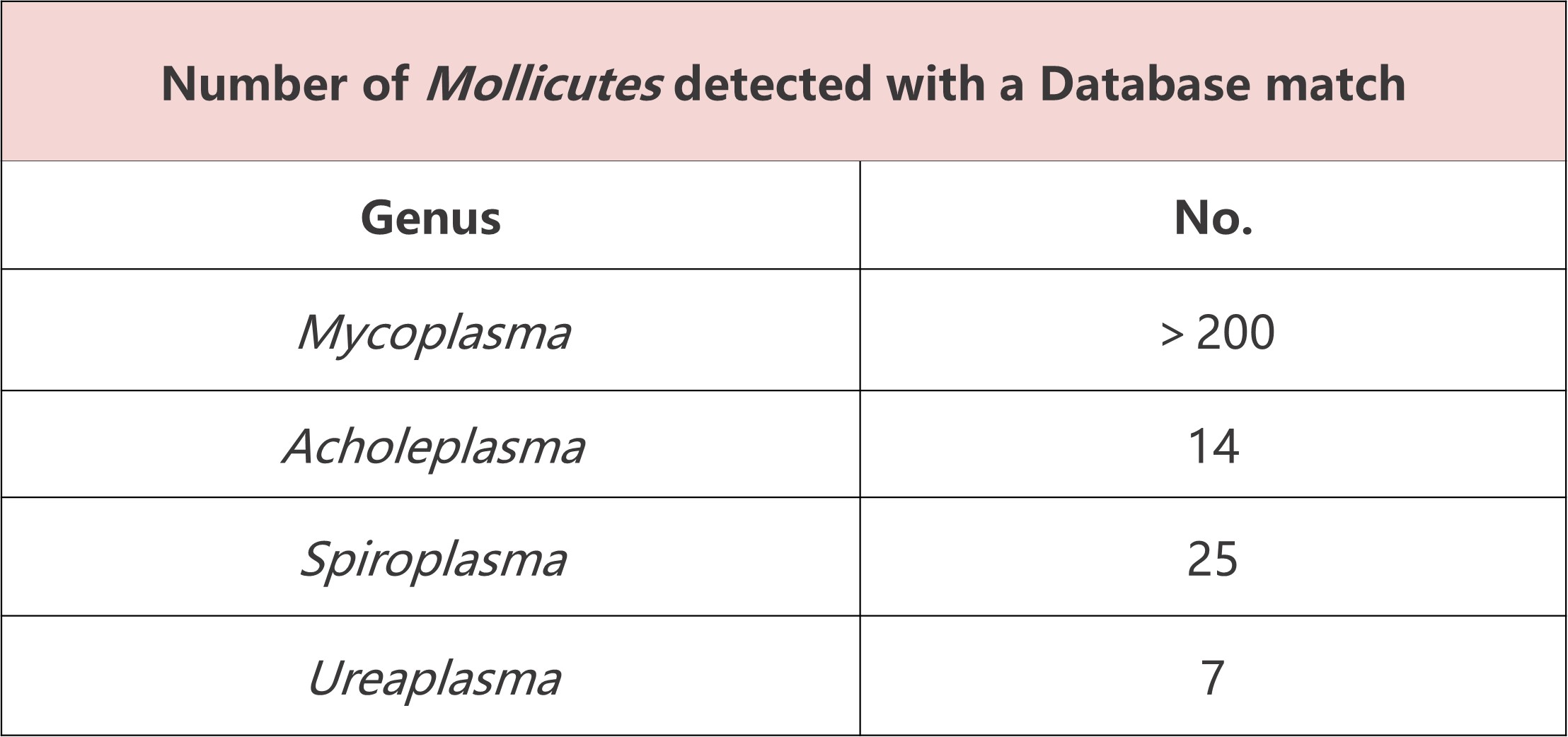
• High Sensitivity: Meets regulatory standards (10 CFU/mL).
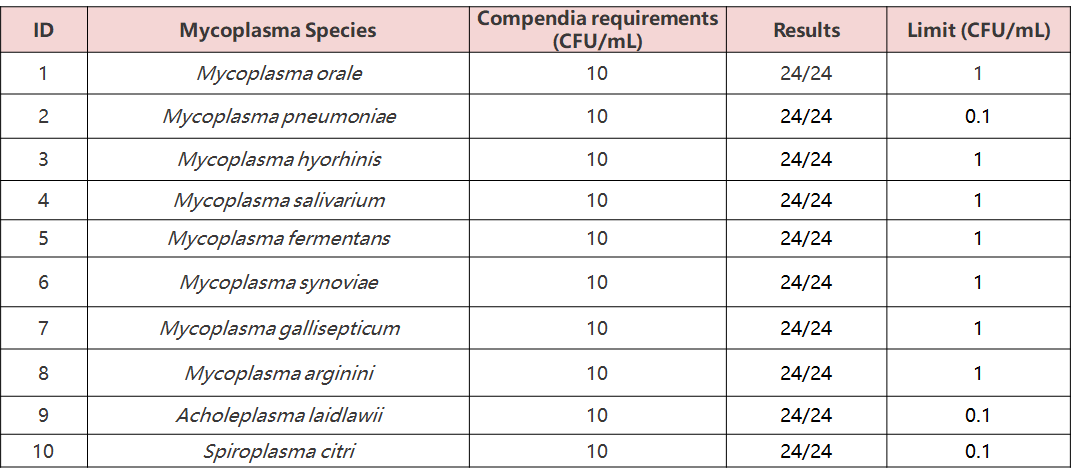
24 tests were conducted on 10 mycoplasma standards (10 CFU/mL), and all results were positive, meeting or exceeding the requirements of EP 2.6.7 (10 CFU/mL).
• Strong Specificity: No cross-reactivity.
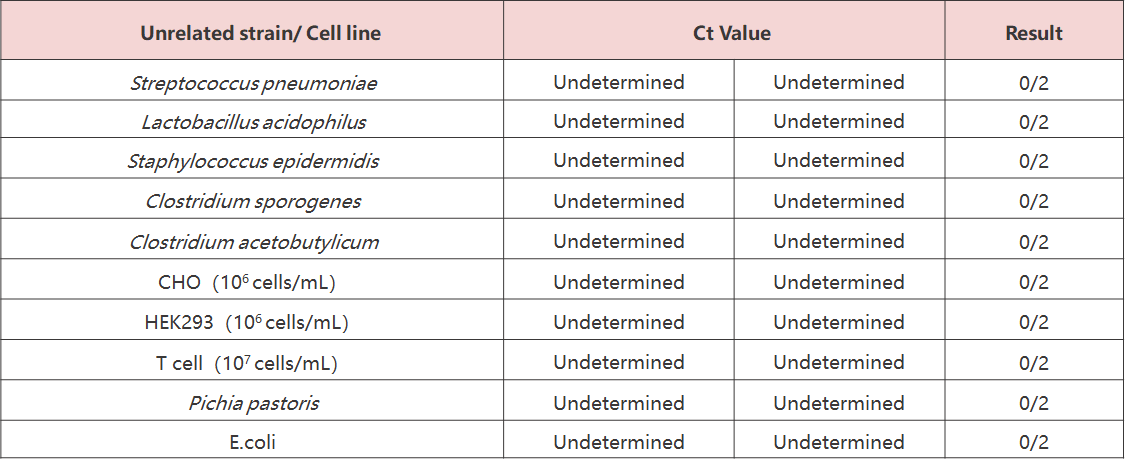
Testing on two common cell lines and four unrelated bacterial strains (Streptococcus pneumoniae, Lactobacillus acidophilus, Staphylococcus epidermidis, and Bacillus subtilis) showed no cross-reactivity, confirming that the test is not influenced by unrelated cells or strains.
1. Rapid Mycoplasma Release Testing: A Critical Safeguard for Biologics Quality-Insights into Cutting-edge Detection Technologies.
2. Unlocking Biosafety: Key Elements and Precise Processes for Rapid Mycoplasma Release Testing.
3. Accelerating Cell Culture and Biopharmaceutical Release with Rapid Mycoplasma Detection-A Comprehensive Guide to Efficient Methods.
4. Overcoming Mycoplasma Release Testing Challenges: The Transition from Traditional to Rapid Methods-A Comparison of Approaches and Their Advantages.
5. Rapid Mycoplasma Release Testing: The "DiscerningEye" for Maintaining Biological Sample Purity-Case Studies.
6. Embracing the Era of Precision Bios detection: Innovation and Application Expansion in Rapid Mycoplasma Release Testing.
7. Excellence in Mycoplasma Release Testing: Meeting Regulatory and Quality Standards-A Deep Dive into Compliance and Industry Benchmarks.
1. Cara N. Wilder, PhD and Yvonne Reid, PhD. Mycoplasma quality control of cell substrates and biopharmaceuticals. ATCC WP-112021-v04.
2. Lesley Young, Julia Sung, Glyn Stacey, and John R Masters. Detection of Mycoplasma in cell cultures. nature protocols. VOL.5 NO.5 (2010) doi:10.1038/nprot.2010.43
3. Phillip Angart, Casey Kohnhorst, Meng-Jung Chiang, and Nilou Sarah Arden. Considerations for risk and control of mycoplasma in bioprocessing. Current Opinion in Chemical Engineering 2018, 22:161–166. doi.org/10.1016/j.coche.2018.09.012
This web search service is supported by Google Inc.







