
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
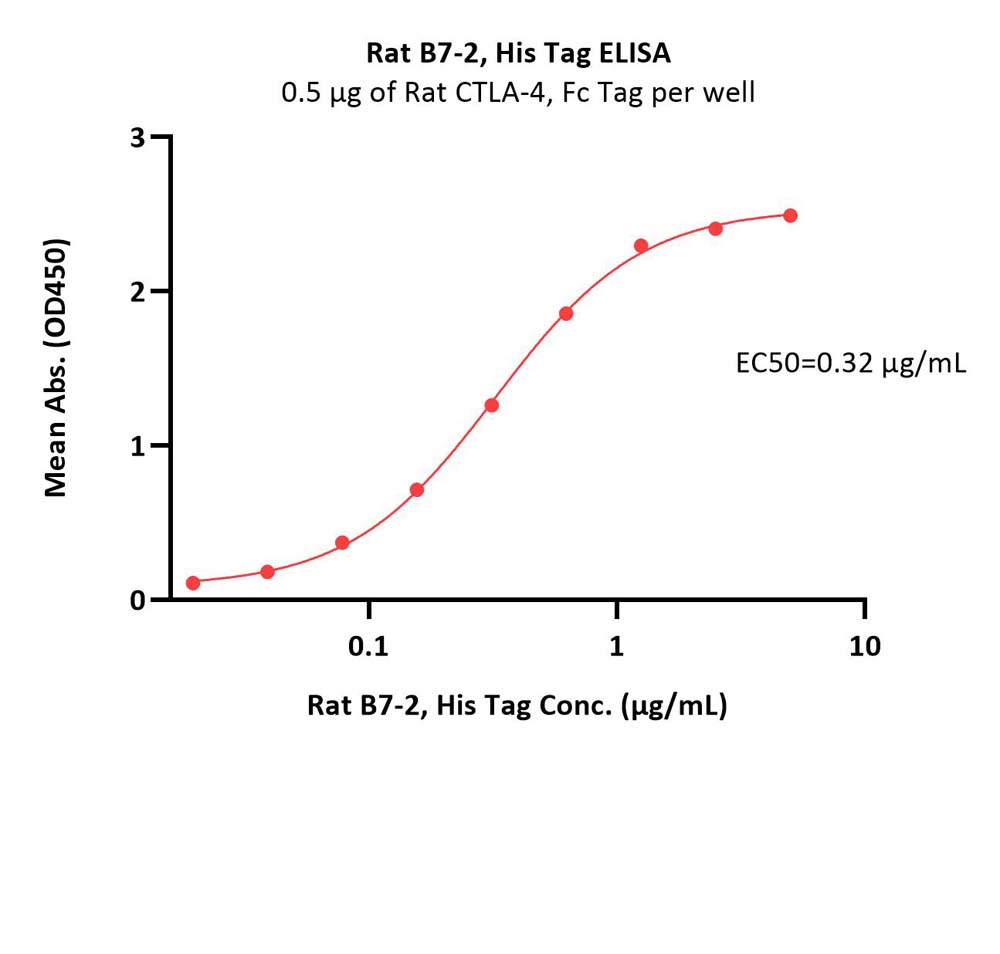
| CD6-R52H9 | Rat | Rat B7-2 / CD86 Protein, His Tag |  |
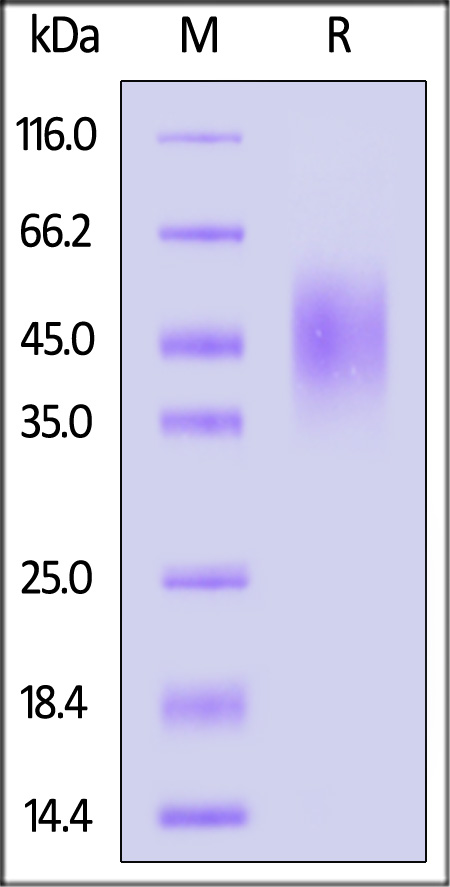
|
|
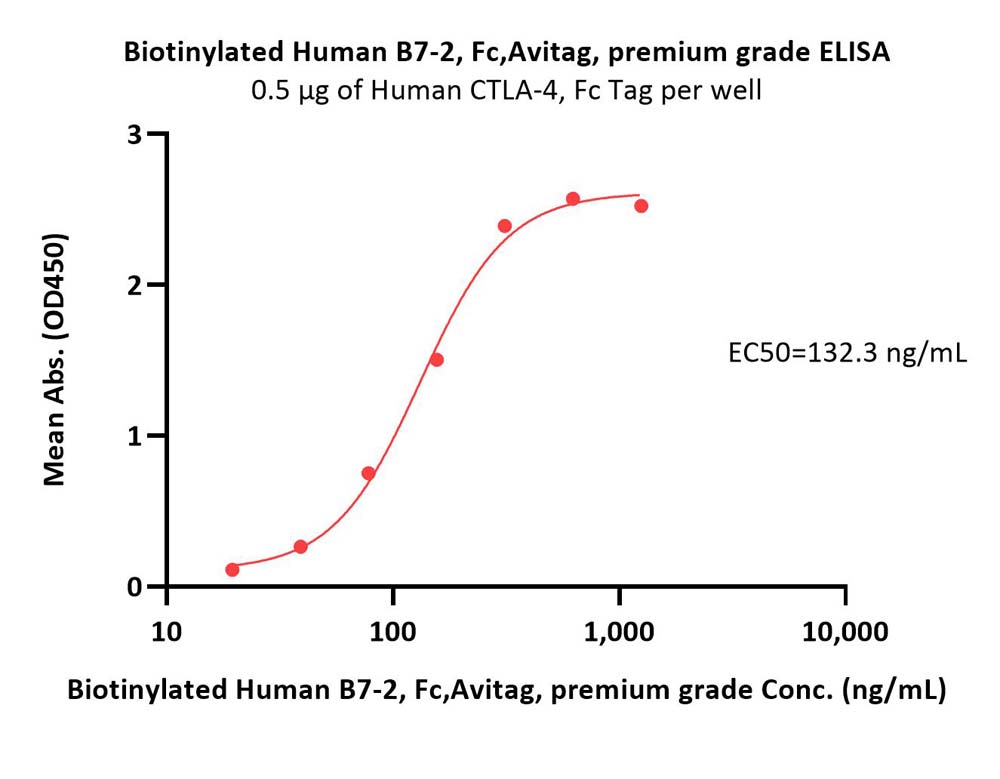
| CD6-H82F5 | Human | Biotinylated Human B7-2 / CD86 Protein, Fc,Avitag™, premium grade |  |
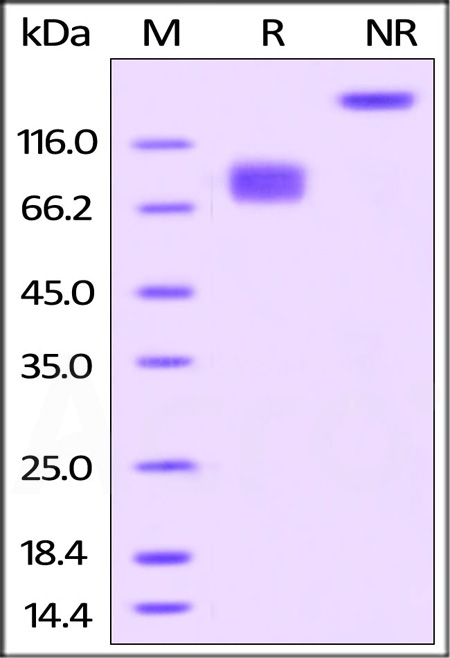
|
|
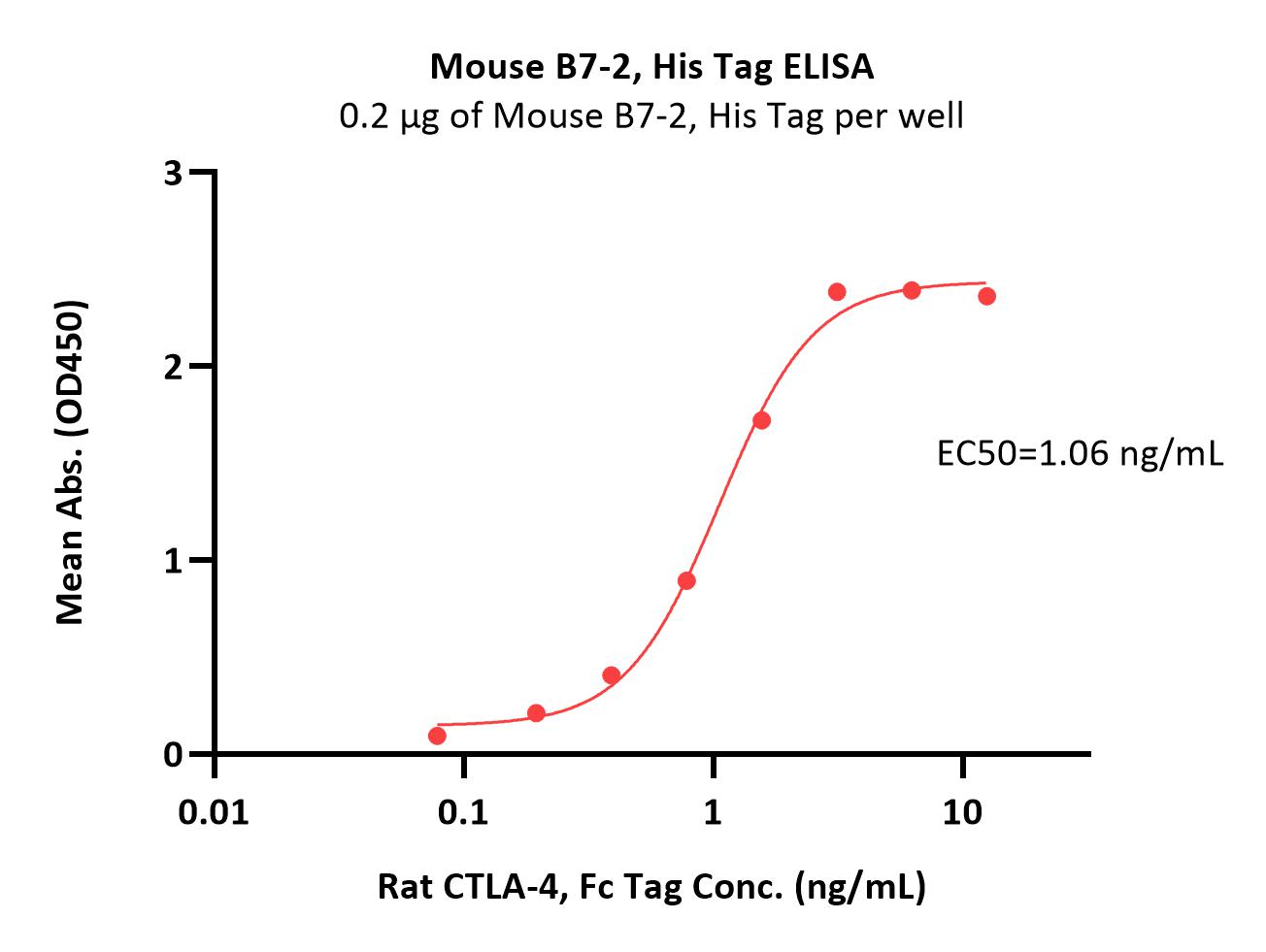
| CD6-M52H0 | Mouse | Mouse B7-2 / CD86 Protein, His Tag (MALS verified) |  |

|
|
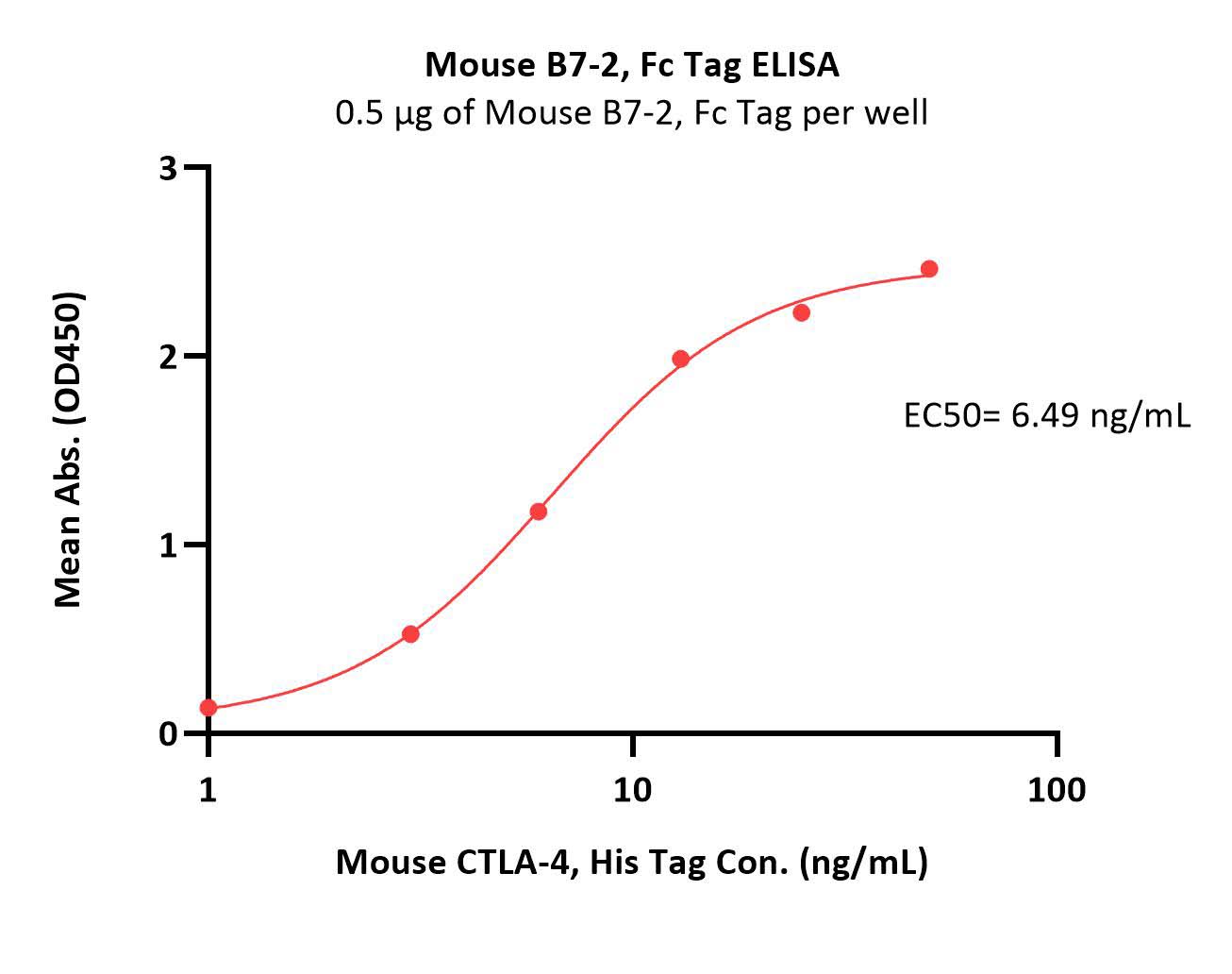
| CD6-M5251 | Mouse | Mouse B7-2 / CD86 Protein, Fc Tag (MALS verified) |  |
|
|
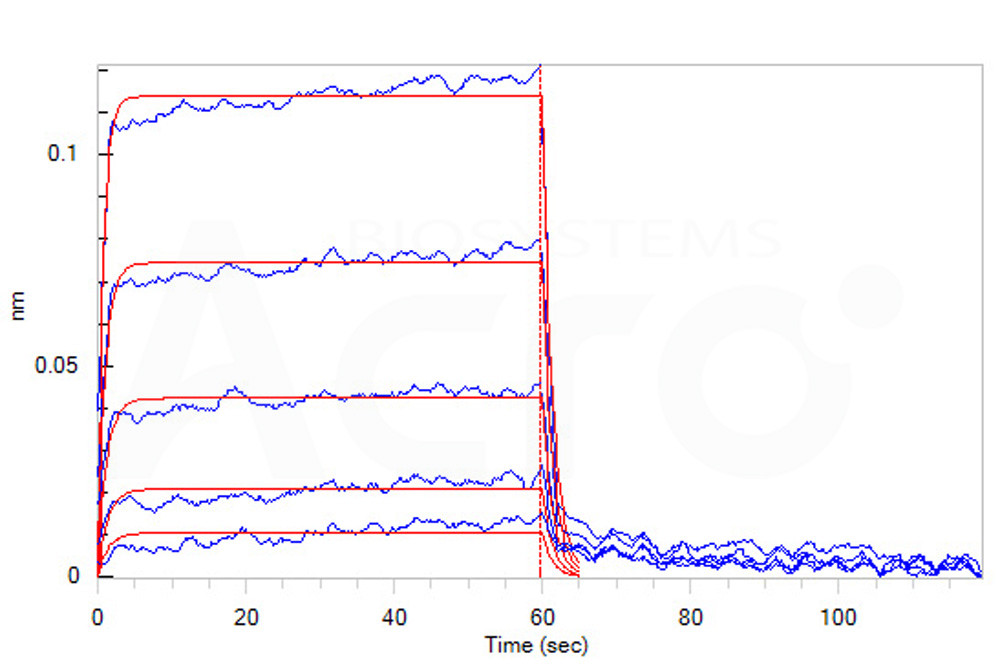
| CD6-C5254 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque B7-2 / CD86 Protein, Fc Tag |  |

|
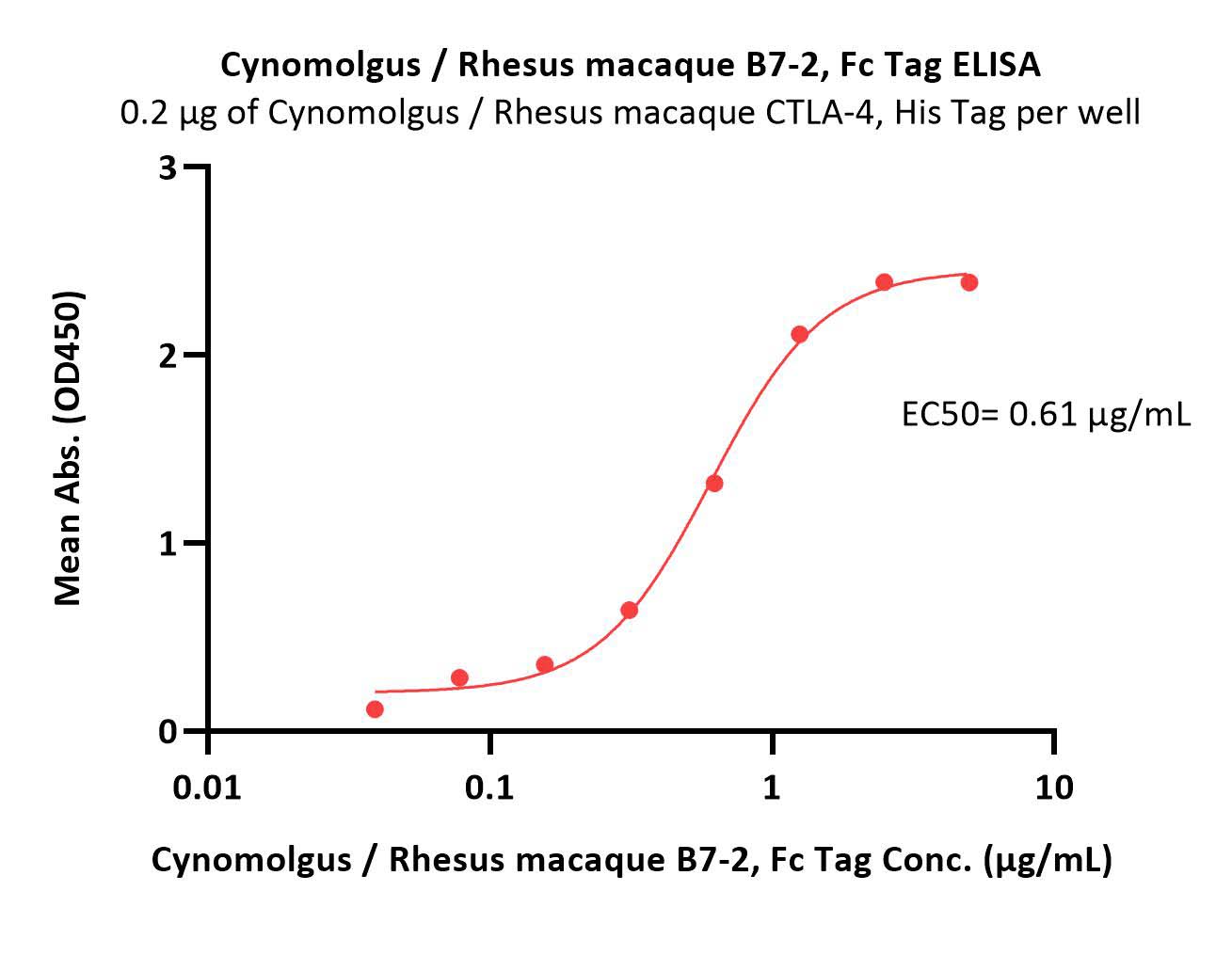
|
| CD6-C52H5 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque B7-2 / CD86 Protein, His Tag (MALS verified) |  |

|
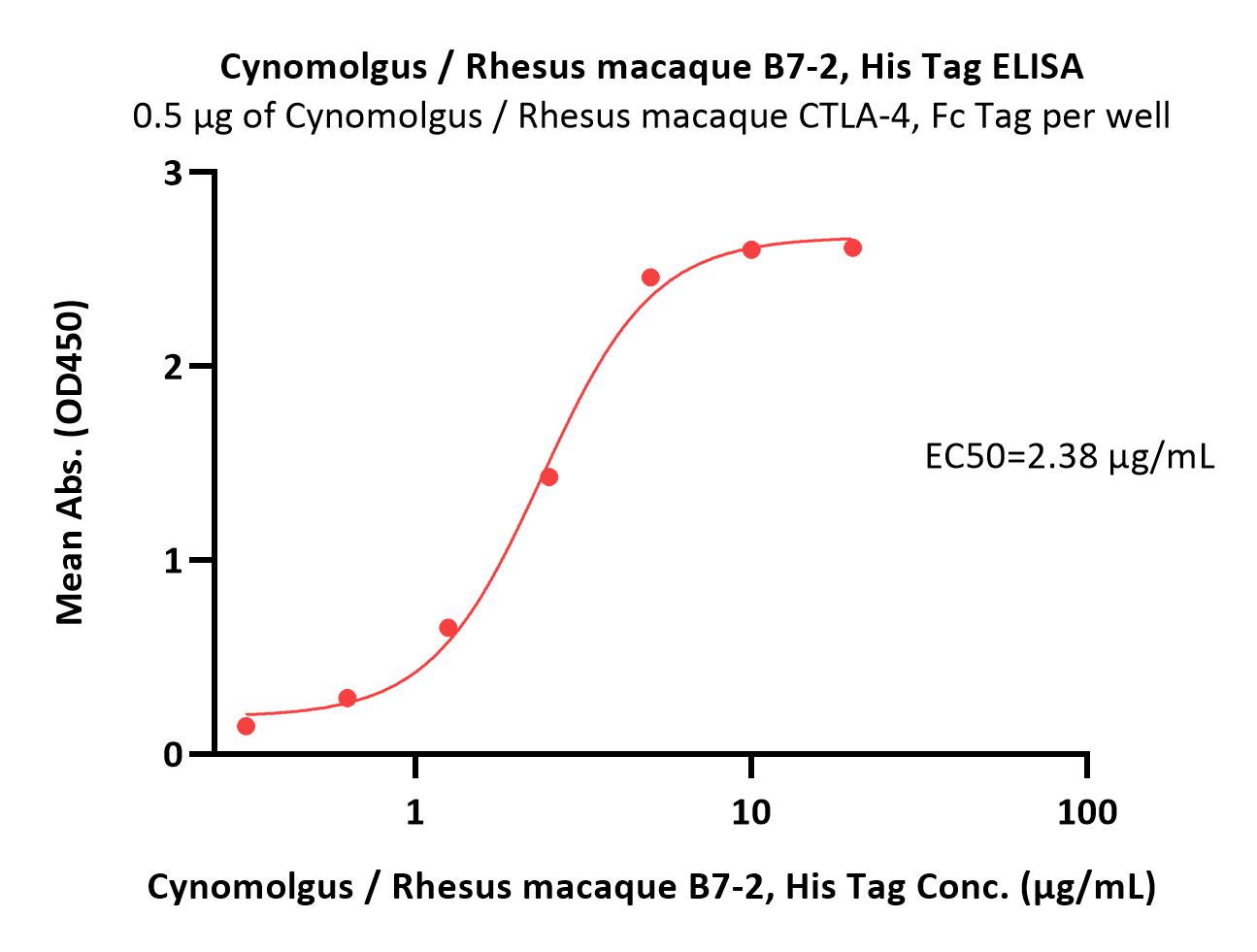
|
| CD6-H5223 | Human | Human B7-2 / CD86 Protein, His Tag (MALS verified) |  |
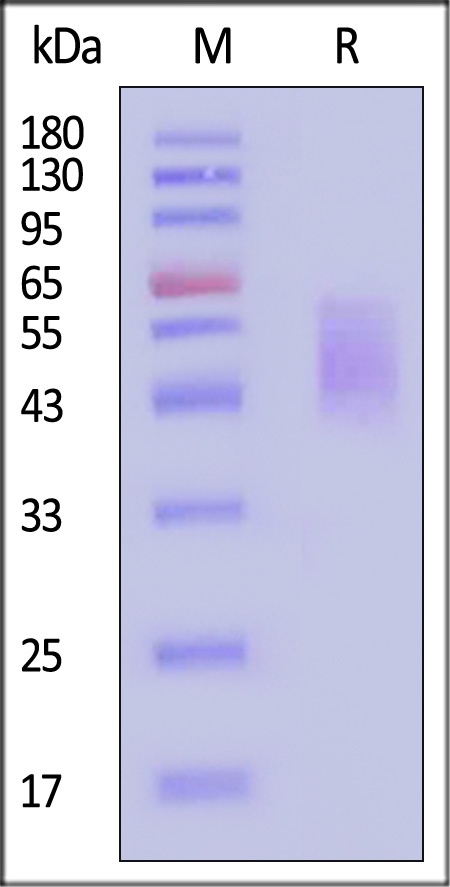
|
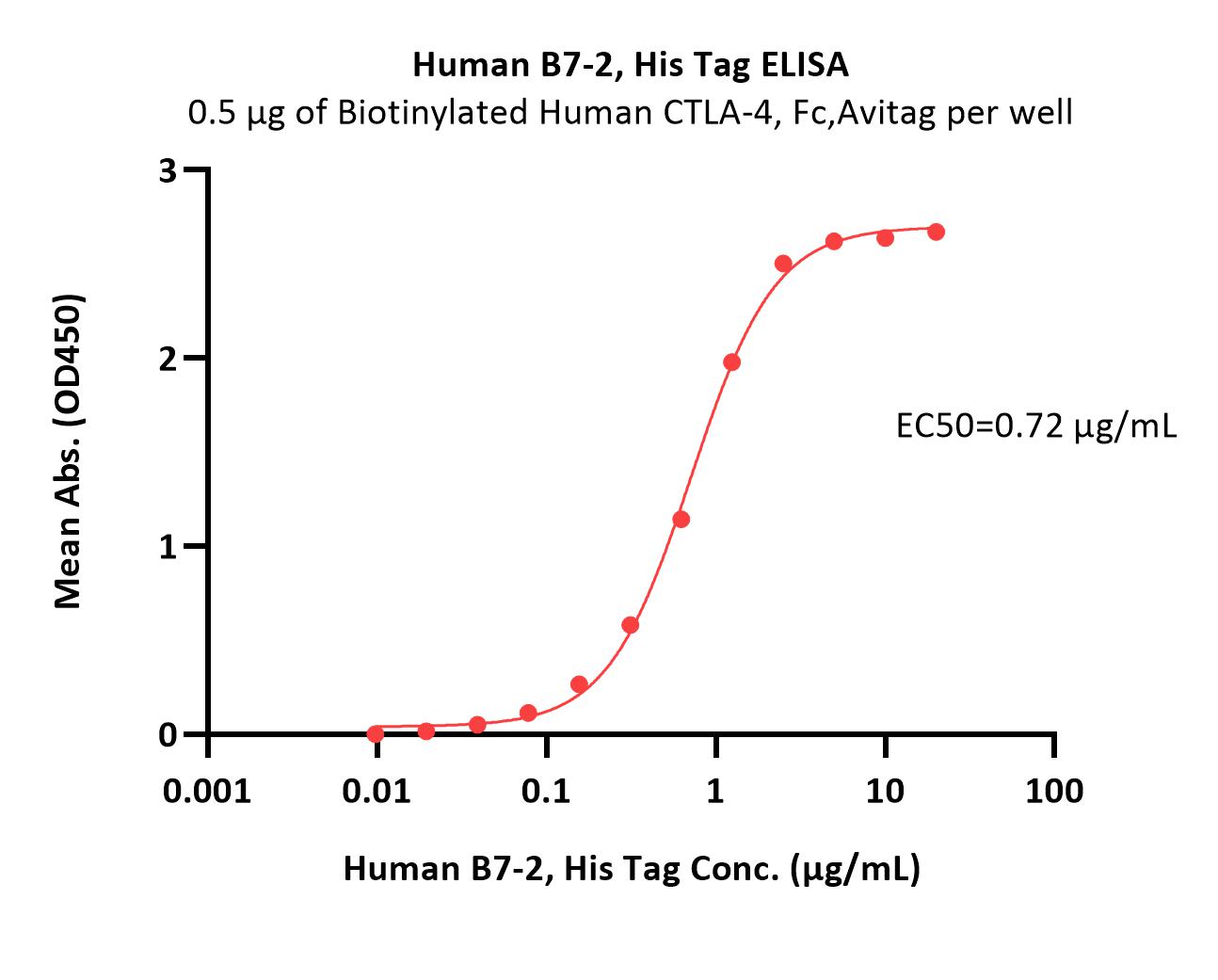
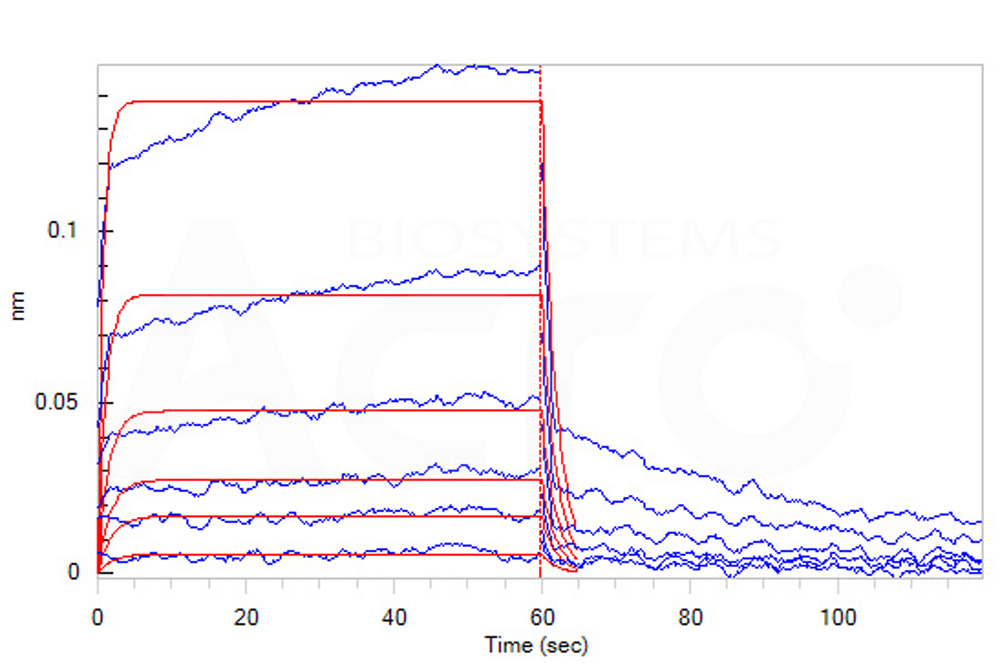
|
| CD6-H5257 | Human | Human B7-2 / CD86 Protein, Fc Tag, premium grade |  |
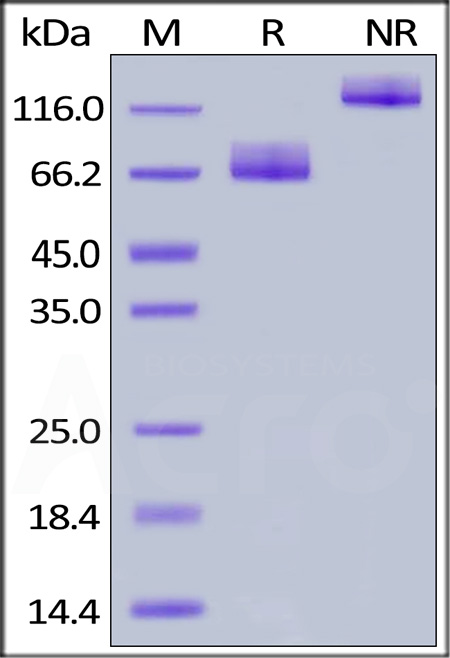
|
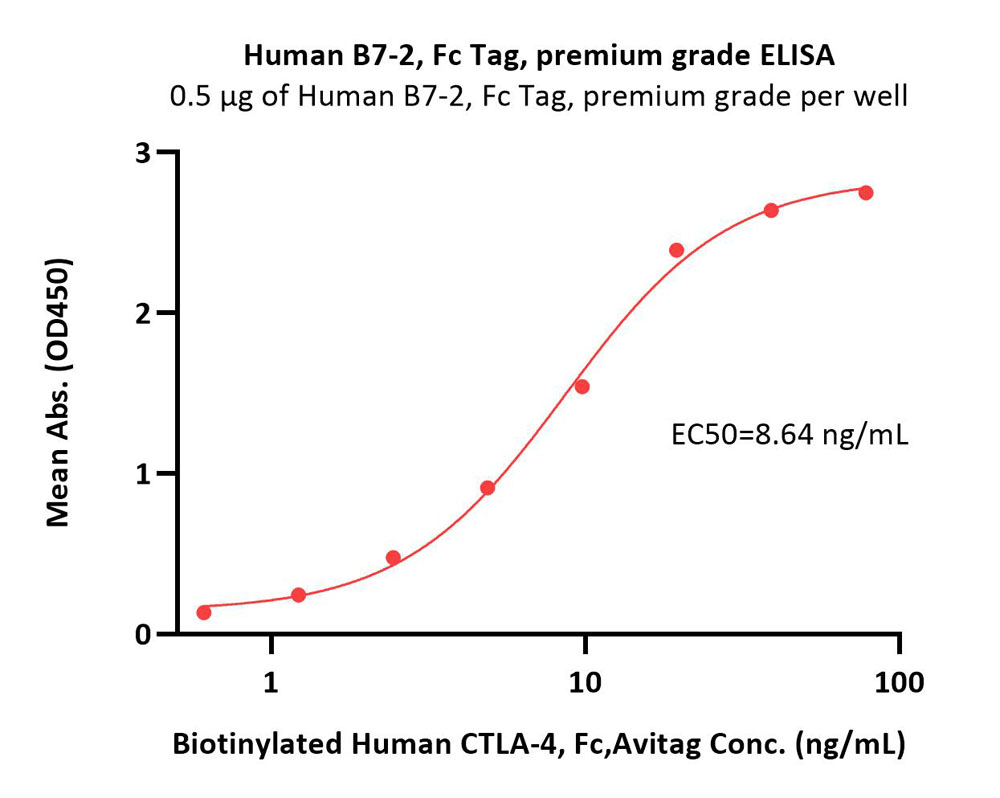
|

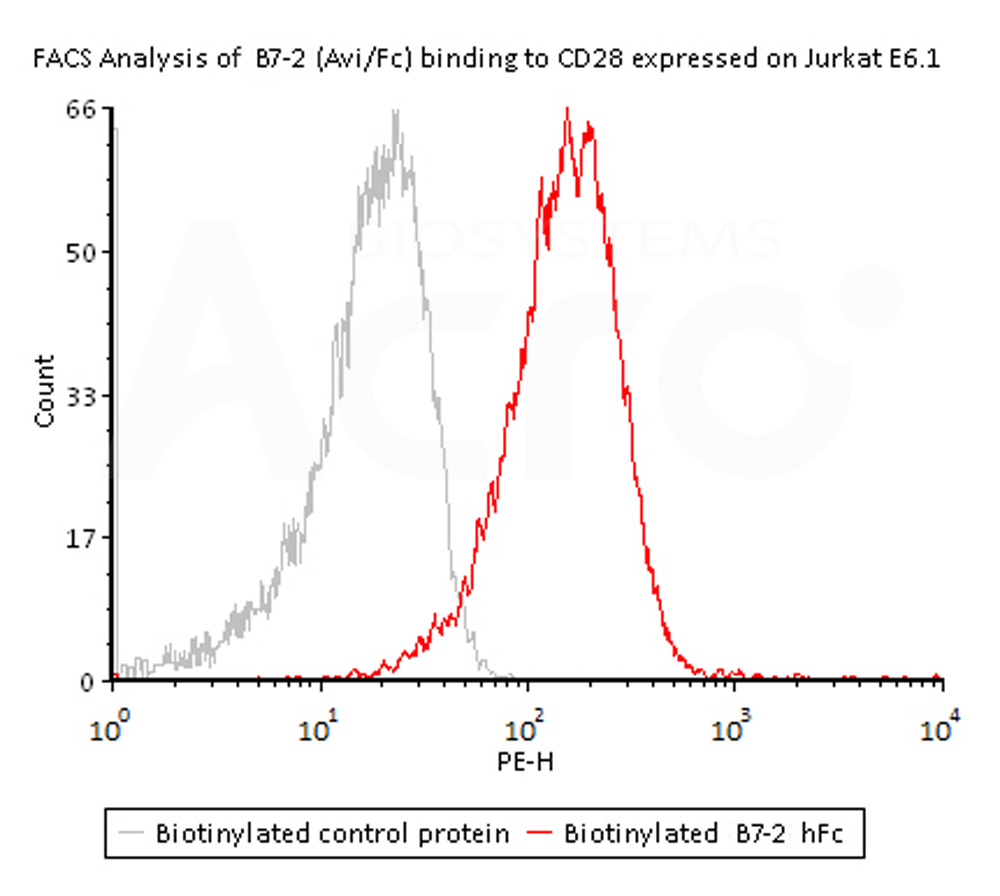
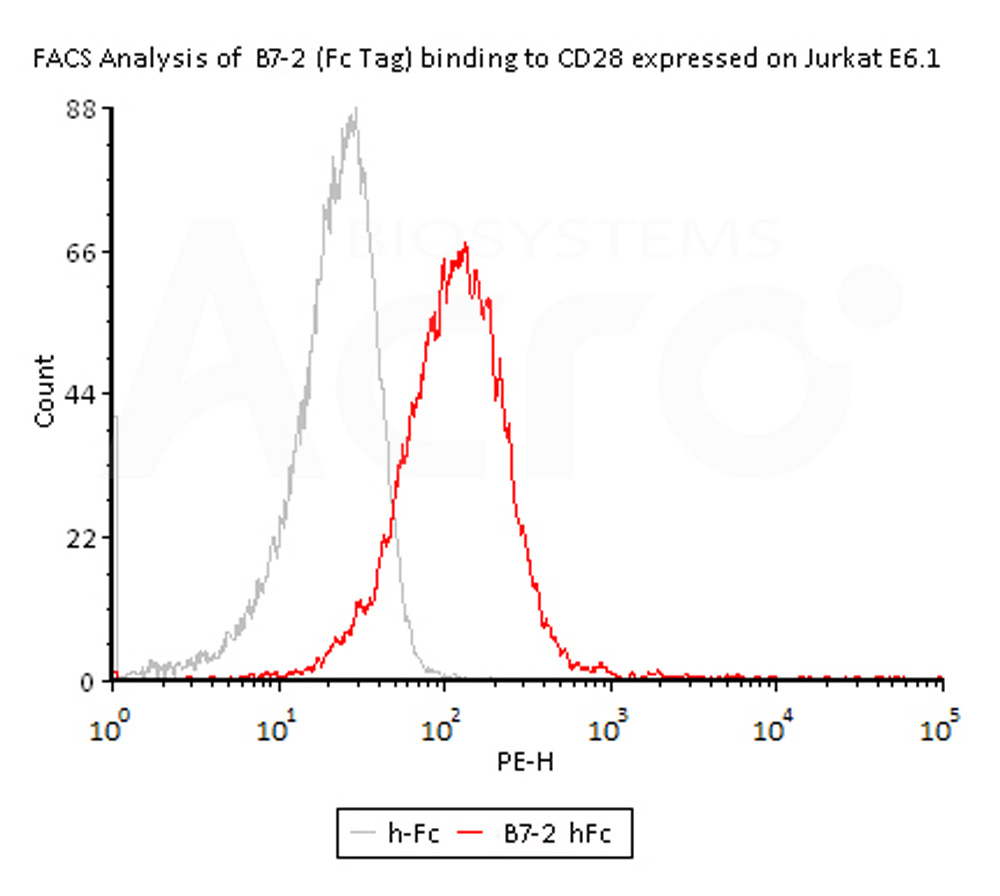
Flow Cytometry assay shows that Biotinylated Human B7-2, Fc,Avitag, premium grade (Cat. No. CD6-H82F5) can bind to CD28 expressed on Jurkat E6.1 cells. The concentration of B7-2 used is 1.5 μg/mL (Routinely tested).
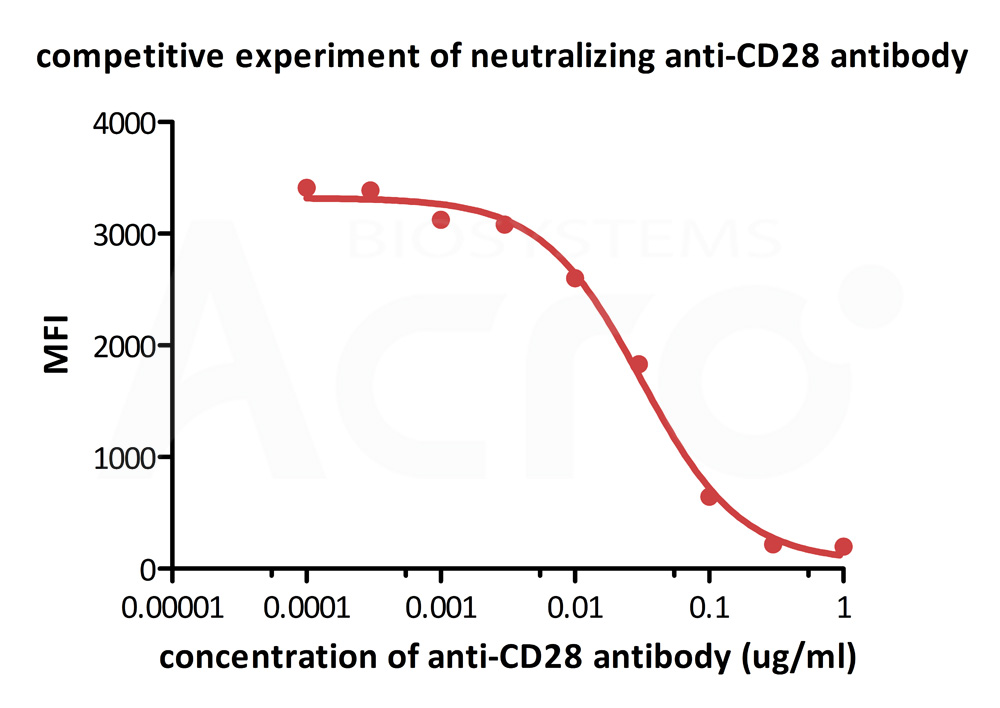
FACS analysis shows that the binding of Biotinylated Human B7-2, Fc,Avitag, premium grade (Cat. No. CD6-H82F5) to CD28 expressed on Jurkat E6.1 was inhibited by increasing concentration of neutralizing Anti-CD28 antibody. The concentration of B7-2 used is 1.5 μg/mL. The IC50 is 0.031 μg/mL (Routinely tested).

Loaded Cynomolgus / Rhesus macaque B7-2, Fc Tag (Cat. No. CD6-C5254) on Protein A Biosensor, can bind Human / Cynomolgus / Rhesus macaque CD28, His Tag with an affinity constant of 11 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Belatacept | LEA29Y; LEA-029; BMS-224818; L104EA29YIg | Approved | Bristol-Myers Squibb Company | Nulojix | United States | Rejection of renal transplantation | Bristol-Myers Squibb Company | 2011-06-15 | Rejection of renal transplantation; Diabetes Mellitus, Type 1; Proteinuria; Pancreatic neuroendocrine tumors (pNET); Arthritis, Rheumatoid; Delayed Graft Function; Rejection of organ transplantation; Rejection in heart transplantation; Kidney Failure, Chronic | Details |
| Abatacept | ONO-4164SC; BMS-188667SC; ONO-4164; BMS-188667 | Approved | Bristol-Myers Squibb Company | Orencia, 恩瑞舒 | United States | Arthritis, Rheumatoid | Bristol-Myers Squibb Company | 2005-12-23 | Anemia, Aplastic; Nephrotic Syndrome; Uveitis; Cardiovascular Diseases; Healthy Aging; Urticaria; Sjogren-Larsson Syndrome; Arthritis, Psoriatic; Colitis, Ulcerative; Arthralgia; Takayasu Arteritis; Lupus Erythematosus, Systemic; Psoriasis; Allergic asthma; Glomerulosclerosis, Focal Segmental; Shwachman-Diamond Syndrome; Mucopolysaccharidosis I; Lymphohistiocytosis, Hemophagocytic; Anemia, Sickle Cell; Thrombasthenia; Kostmann Syndrome; Behcet Syndrome; Vitiligo; Crohn Disease; Muscular Diseases; Granulomatous Disease, Chronic; Lung Diseases, Interstitial; Leukocyte-Adhesion Deficiency Syndrome; Inflammation; Common Variable Immunodeficiency; Myocarditis; Eye Diseases; Scleroderma, Diffuse; Arthritis; Hematologic Diseases; Coronavirus Disease 2019 (COVID-19); Myositis; Alopecia Areata; Immunoglobulin G4-Related Disease; Dermatomyositis; Giant Cell Arteritis; Diabetes Mellitus, Type 1; Polymyositis; Arthritis, Rheumatoid; Wiskott-Aldrich Syndrome; Myasthenia Gravis; Hematologic Neoplasms; Polychondritis, Relap | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ORCA-010 | ORCA-010 | Phase 2 Clinical | Orca Therapeutics Bv | Prostatic Neoplasms | Details |
| Chimeric antigen receptor T-cell therapy (Hunan Zhaotai Yongren Biotech) | Z-CTLs | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| VDJ-142 | VDJ-142 | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid; Lupus Erythematosus, Systemic; Inflammation | Details |
| Abatacept biosimilar (Dr. Reddy's Laboratories) | DRL-AB | Phase 1 Clinical | Dr.Reddy's Laboratories Ltd | Arthritis, Rheumatoid | Details |
| Recombinant human CTLA-4-FC fusion protein(Beijing Vdjbio) | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid | Details |
This web search service is supported by Google Inc.







