
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
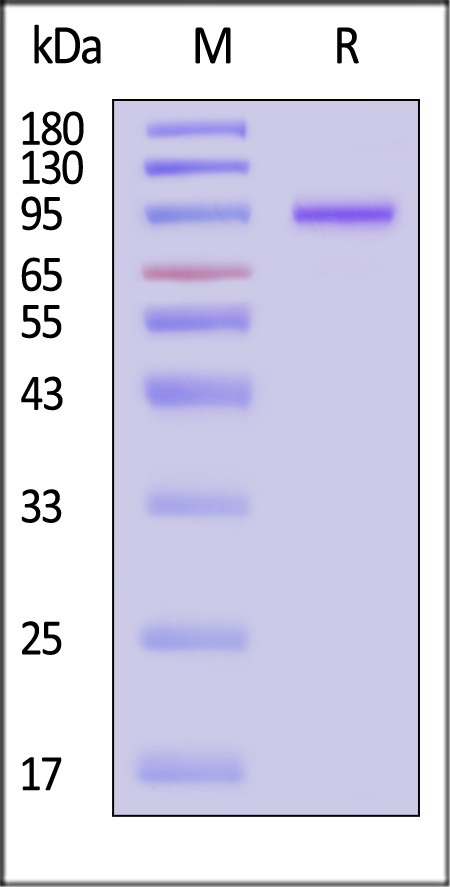
| VP1-A5147 | Adeno-associated virus 9 | AAV9 VP1, Recombinant Protein | 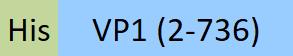 |
|
|
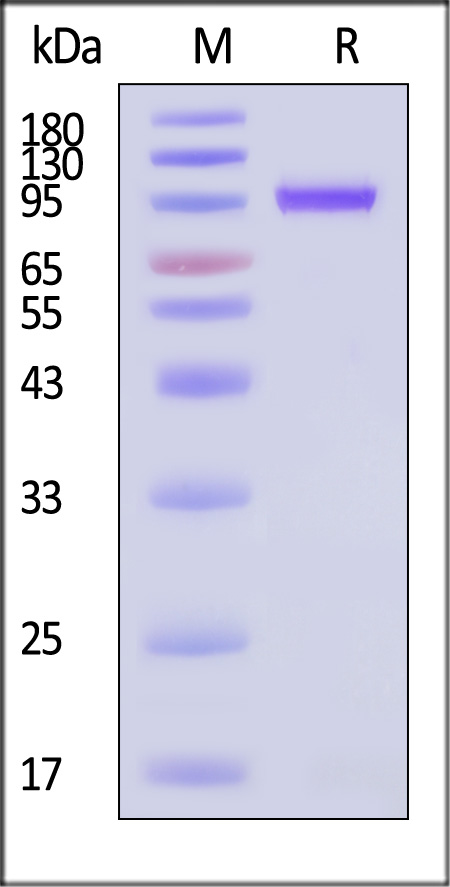
| VP1-A5146 | Adeno-associated virus 8 | AAV8 VP1, Recombinant Protein | 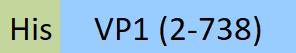 |
|
|
| VP1-A5145 | Adeno-associated virus 6 | AAV6 VP1, Recombinant Protein | 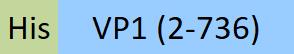 |
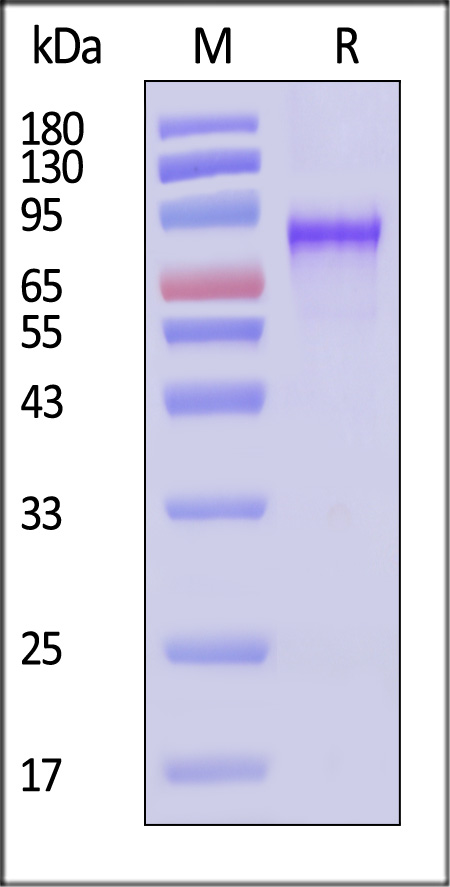
|
|
| VP1-A5144 | Adeno-associated virus 5 | AAV5 VP1, Recombinant Protein | 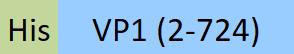 |
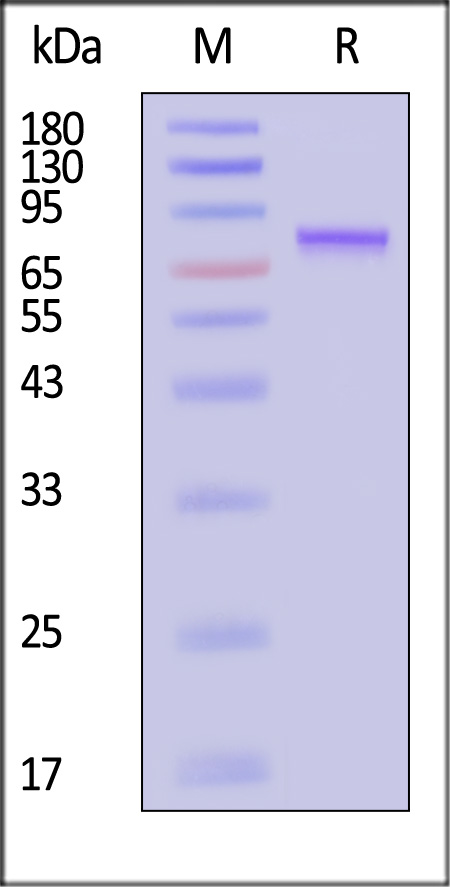
|
|
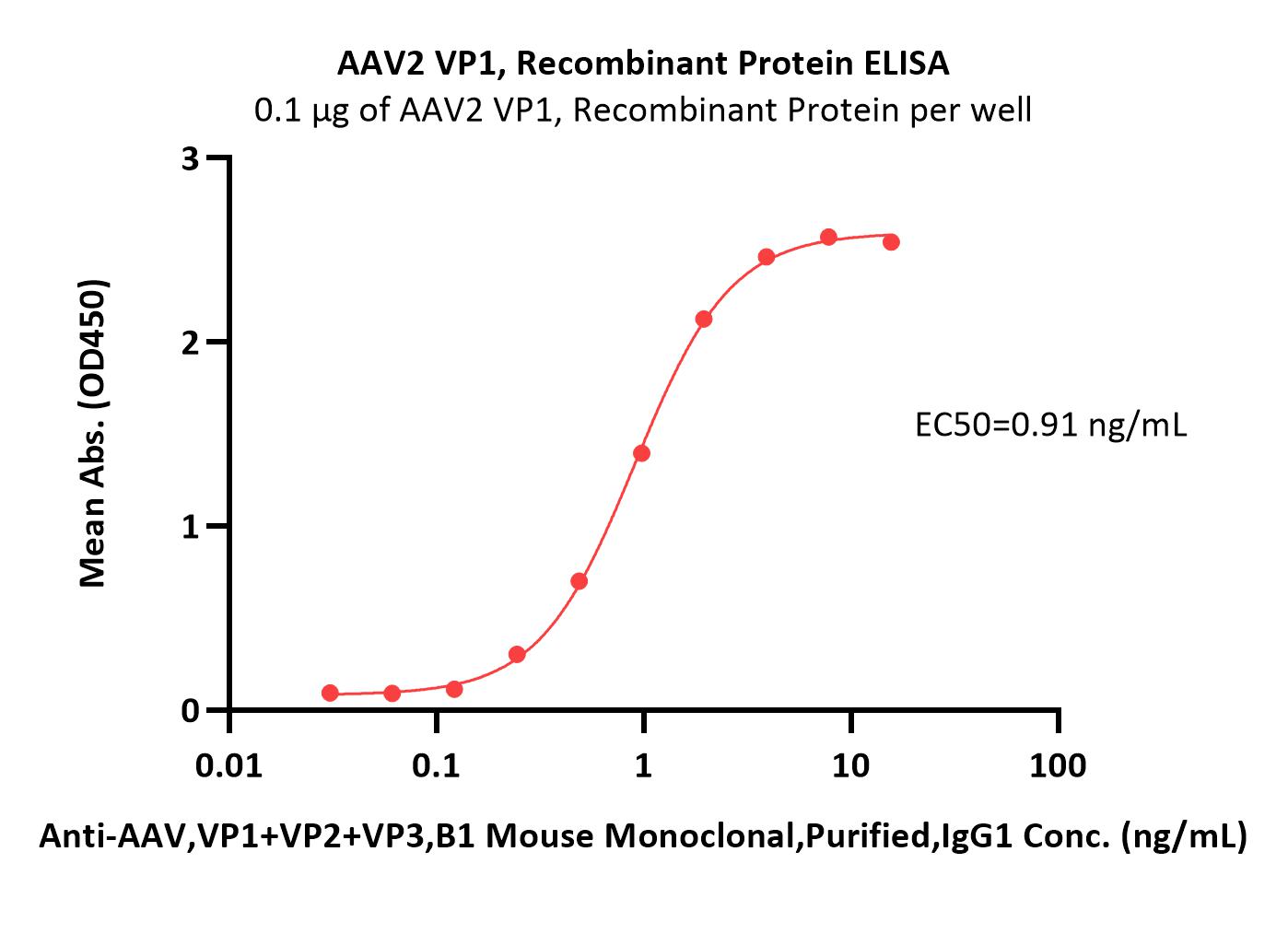
| VP1-A5143 | Adeno-associated virus 2 | AAV2 VP1, Recombinant Protein |  |
|
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenacapavir sodium | GS-6207 | Approved | Gilead Inc | SUNLENCA | EU | HIV Infections | Gilead Sciences Ireland Uc | 2022-08-17 | HIV Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Morphothiadine Mesylate | GLS4JHS; GLS-4 | Phase 3 Clinical | South China Center For Innovative Pharmaceuticals, Guangdong Dongyangguang Pharmaceutical Co Ltd | Hepatitis B, Chronic; Hepatitis B | Details |
| GS-1720/GS-4182 | GS-1720/GS-4182 | Phase 3 Clinical | Gilead Sciences Inc | HIV Infections | Details |
| Bictegravir/Lenacapavir sodium | Phase 3 Clinical | Gilead Sciences Inc | HIV Infections | Details | |
| GS-4182 | GS-4182 | Phase 3 Clinical | Gilead Sciences Inc | HIV Infections | Details |
| Lenacapavir/Islatravir | MK-8591D | Phase 3 Clinical | Merck & Co Inc, Gilead Sciences Inc | HIV Infections | Details |
| Azodicarbonamide | HPH-116; NSC-674447 | Phase 2 Clinical | H-Phar Pharmaceuticals | HIV Infections | Details |
| QL-007 | QL-007 | Phase 2 Clinical | Qilu Pharmaceutical Co Ltd | Hepatitis B, Chronic | Details |
| GST-HG141 | GST-HG141 | Phase 2 Clinical | Fujian Cosunter Pharmaceutical Co Ltd | Hepatitis B, Chronic; Hepatitis B | Details |
| bersacapavir | JNJ-56136379; JNJ-379; JNJ-6379; 14C JNJ-56136379 | Phase 2 Clinical | Johnson & Johnson | Hepatitis, Chronic; Hepatitis B, Chronic; Renal Insufficiency; Hepatitis B; Hepatic Insufficiency | Details |
| RO-7049389 | RO-7049389 | Phase 2 Clinical | F. Hoffmann-La Roche Ltd | Hepatitis B, Chronic | Details |
| Traxivitug | MAU-868 | Phase 2 Clinical | Novartis Pharma Ag | BK Polyomavirus Infections; Polyomavirus Infections | Details |
| Vebicorvir | ABI-H0731; ABIH-0731 | Phase 2 Clinical | Indiana University | Hepatitis B, Chronic; Hepatitis B | Details |
| Canocapavir | ZM-H1505R; CB-001; CB-01 | Phase 2 Clinical | Shanghai Zhimeng Biopharma Inc | Hepatitis B, Chronic; Hepatitis B | Details |
| VH-4011499 | VH-4011499; GSK-4011499; VH-4011499-A; VH-499 | Phase 2 Clinical | Viiv Healthcare | HIV Infections | Details |
| VH-4004280 | VH-4004280; VH 4004280A | Phase 2 Clinical | Viiv Healthcare | HIV Infections | Details |
| Firzacorvir | 2158; ABI-2158; ABI-H2158 | Phase 2 Clinical | Assembly Biosciences Inc, Indiana University | Hepatitis B, Chronic | Details |
| BIT-225 | BIT-225 | Phase 2 Clinical | Biotron | HIV Infections; Hepatitis C | Details |
| Fipravirimat | BMS-986197; GSK-3640254 | Phase 1 Clinical | Bristol-Myers Squibb Company | HIV Infections | Details |
| AB-423 | AB-423 | Phase 1 Clinical | Arbutus Biopharma Corp | Hepatitis B | Details |
| H-05(Protheragen) | Phase 1 Clinical | Protheragen Inc | Hepatitis B | Details | |
| EV68-228-N | EVD68-228N; EVD68-228-N; EV68-228-N; KB-13-A | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), KBio Inc | Enterovirus Infections; Acute Flaccid Myelitis | Details |
| PLD-401 | PLD-401 | Phase 1 Clinical | Pelemed Co Ltd | Hepatitis B, Chronic | Details |
| Morphothiadine Mesylate/Ritonavir | Phase 1 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Hepatitis B, Chronic | Details | |
| LW-231 | LW-231 | Phase 1 Clinical | Shanghai Longwood Biopharmaceuticals Co Ltd | Hepatitis B, Chronic | Details |
| ALG-010184 | ALG-010184 | Phase 1 Clinical | Aligos Therapeutics Inc | Hepatitis B, Chronic | Details |
| ALG-000184 | ALG-000184 | Phase 1 Clinical | Aligos Therapeutics Inc | Hepatitis B, Chronic | Details |
| XT-1061 | XTYW-001; XTYW001; XT-1061; XT1061 | Phase 1 Clinical | Xi'an Xintong Pharmaceutical Research Co Ltd | Hepatitis B, Chronic | Details |
| GS-CA2 | GS-CA2; GS-CA-2 | Phase 1 Clinical | Gilead Sciences Inc | HIV Infections | Details |
This web search service is supported by Google Inc.







