
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| NP1-C52H3 | Cynomolgus | Cynomolgus NPR1 / NPRA Protein, His Tag (MALS verified) |

|
||
| NP1-M52H3 | Mouse | Mouse NPR1 / NPRA Protein, His Tag (MALS verified) |

|
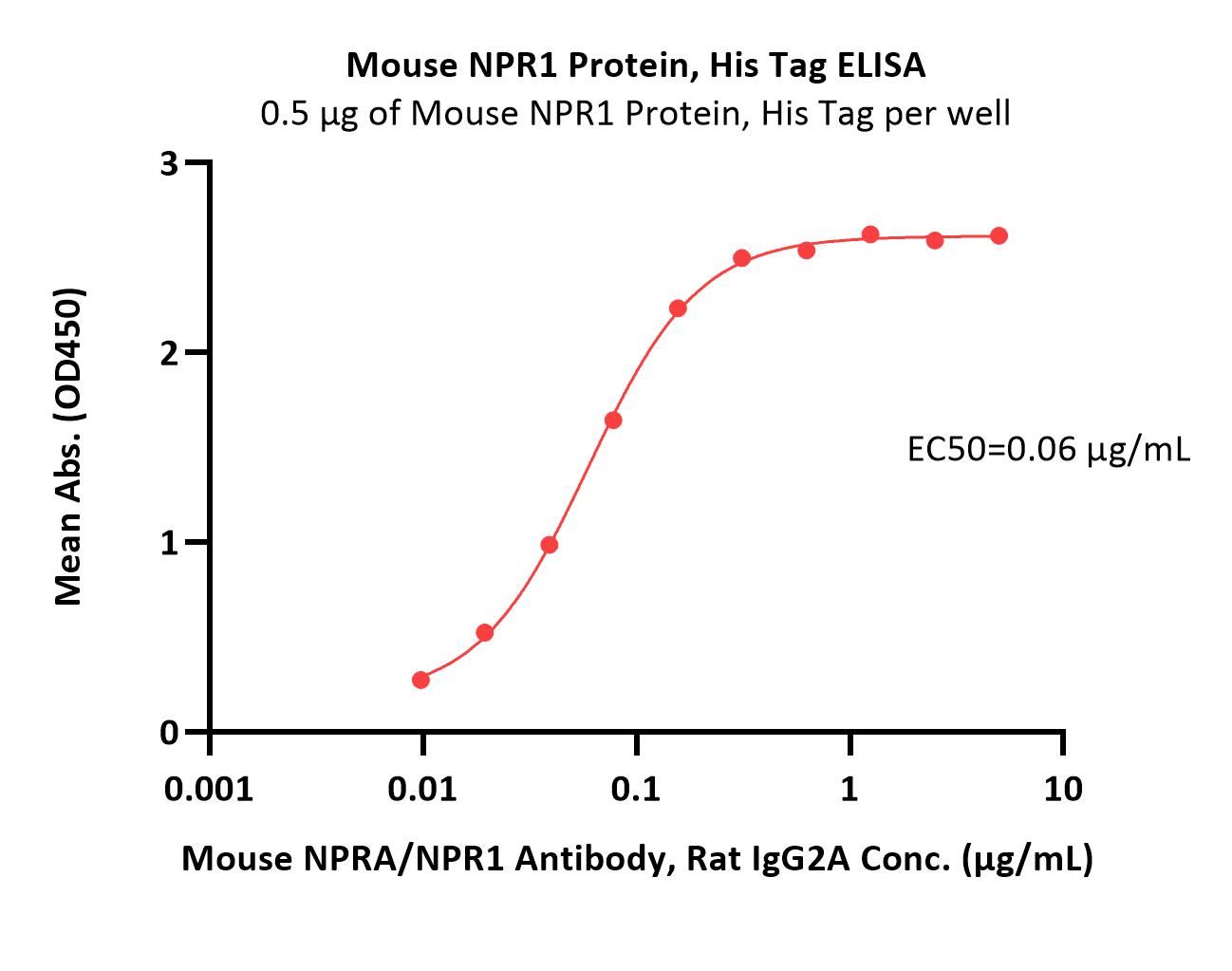
|
|
| NP1-H82E9 | Human | Biotinylated Human NPR1 / NPRA Protein, His,Avitag™ (MALS verified) |  |

|
|
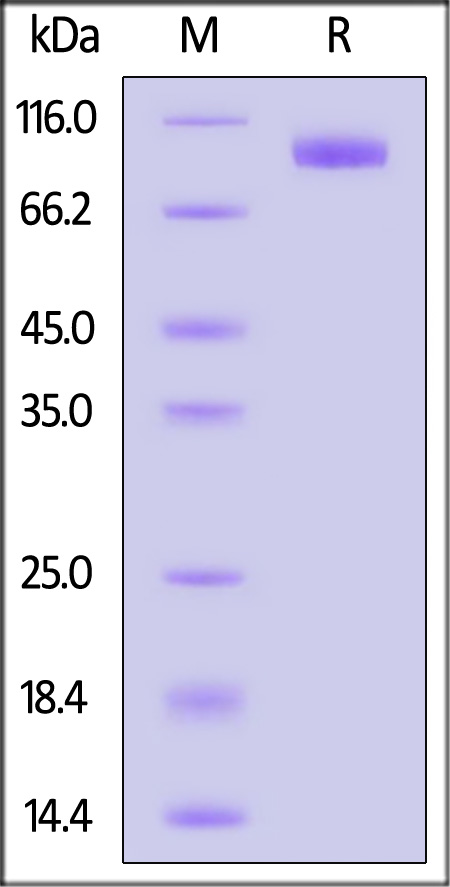
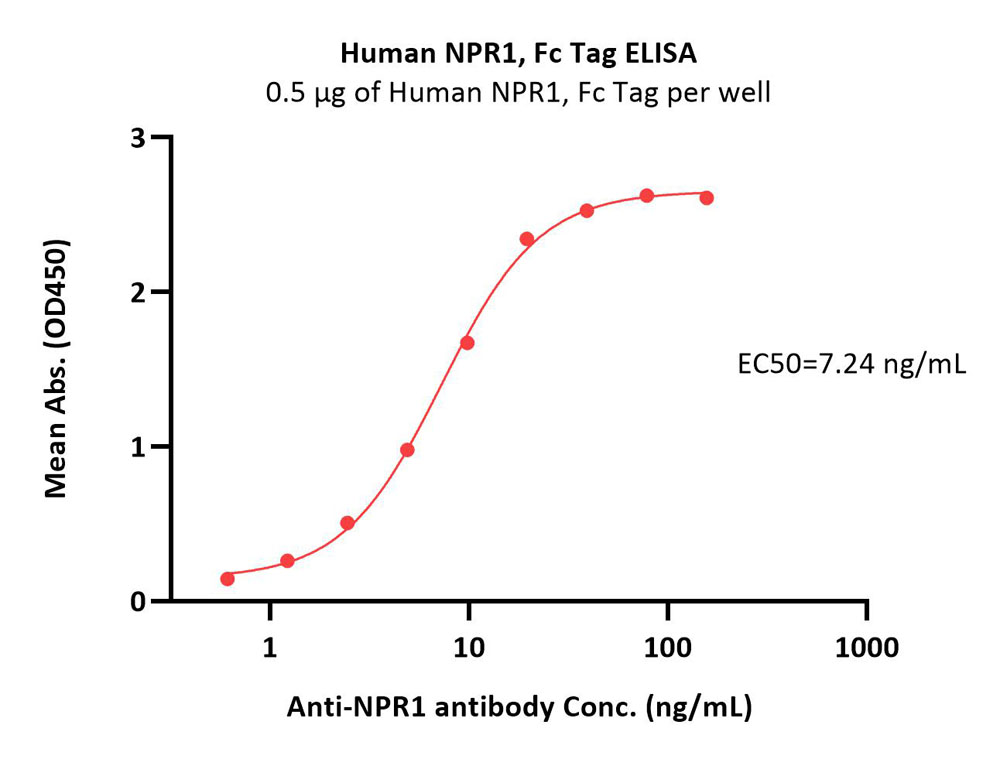
| NP1-H5259 | Human | Human NPR1 / NPRA Protein, Fc Tag (MALS verified) |  |
|
|

Immobilized Human NPR1, Fc Tag (Cat. No. NP1-H5259) at 5 μg/mL (100 μL/well) can bind Anti-NPR1 antibody with a linear range of 0.6-20 ng/mL (Routinely tested).

The purity of Cynomolgus NPR1 Protein, His Tag (Cat. No. NP1-C52H3) is more than 85% and the molecular weight of this protein is around 125-155 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Recombinant human brain natriuretic peptide (Tibet Rhodiola) | Approved | Tibet Rhodiola Pharmaceutical Holding Company | 新活素 | Mainland China | Heart Failure | Chengdu Rhodiola Bio-Pharmaceutical Co Ltd | 2005-04-11 | Heart Failure; Acutely decompensated congestive heart failure | Details | |
| Sodium Nitroprusside | SNP | Approved | F. Hoffmann-La Roche Ltd | Nipride, Nitriate, Nitropress | Mainland China | Heart Failure; Hypertension | China Resources Double-Crane Pharmaceutical Co Ltd | 1974-05-10 | Hypotension; Schizophrenia; Heart Failure; Myocardial Infarction; Hypertension; Healthy Aging; Pulmonary Edema | Details |
| Carperitide | SUN-4936; SUN-4936h; SUN-4936r; SUN-Y4936r; alpha-hANP; ANF (99-126); ANP (99-126); CDD (99-126) | Approved | Daiichi Sankyo Co Ltd | HANP | Japan | Heart Failure | Daiichi Sankyo Co Ltd | 1995-01-20 | Heart Failure; Respiratory Distress Syndrome, Adult; Cardiomyopathies | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Recombinant human brain natriuretic peptide (Shanghai Jiao Tong University) | Phase 3 Clinical | Shanghai Jiaotong University | Tetralogy of Fallot; Heart Defects, Congenital | Details | |
| Nesiritide | BNP-32 (human); JNS-004; hBNP; SC-70400 | Phase 2 Clinical | Johnson & Johnson | Myocardial Ischemia; Hyperemia; Heart Failure; Renal Insufficiency; Acutely decompensated congestive heart failure; Sleep Disorders, Circadian Rhythm; Dyspnea; Hypertension; Coronary Disease; Cardiomyopathies; Cardiovascular Diseases; Hyperinsulinism; Obesity; Heart Failure, Systolic; Diabetes Mellitus | Details |
| REGN-7544 | REGN-7544; REGN7544 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Hypotension; Postural Orthostatic Tachycardia Syndrome | Details |
| XXB-750 | XXB-750 | Phase 2 Clinical | Novartis Pharma Ag | Heart Failure; Hypertension; Heart Failure, Systolic | Details |
| GNP | Phase 2 Clinical | Guangzhou Leien Kangya Biomedical Technology Co Ltd | Heart Failure; Acutely decompensated congestive heart failure | Details | |
| REGN-5381 | REGN-5381 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Heart Failure | Details |
| Recombinant human brain natriuretic peptide(Shijiazhuang wotai) | Phase 1 Clinical | Shijiazhuang wotai Biotechnology Co Ltd | Heart Failure; Acutely decompensated congestive heart failure | Details |
This web search service is supported by Google Inc.







