
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































Flow cytometric analysis of Raji cells staining with PE-Labeled Human CTLA-4 Protein, His Tag (Cat. No. CT4-HP2H3) at 1:50 dilution (2μL of the antibody stock solution corresponds to labeling of 5e5 cells in a final volume of 100 µL), compared with negative control protein. PE signal was used to evaluate the binding activity (QC tested).
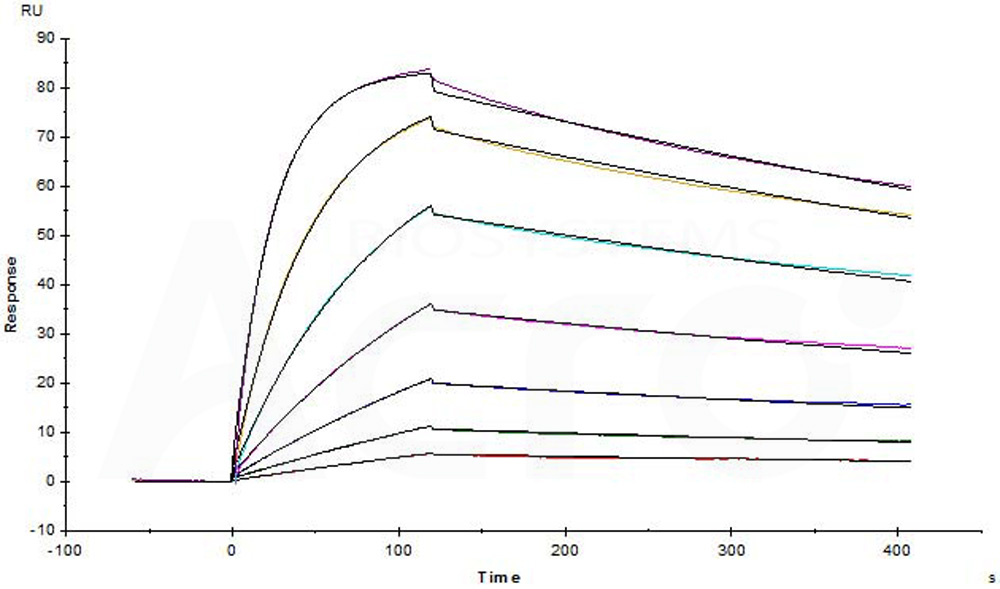
Biotinylated Human CTLA-4, His,Avitag (Cat. No. CT4-H82E3) captured on Biotin CAP - Series S sensor Chip can bind Yervoy (Ipilimumab) with an affinity constant of 0.635 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Cadonilimab | AK-104; AK104 | Approved | Zhongshan Akeso Biopharma Co Ltd | 开坦尼 | Mainland China | Uterine Cervical Neoplasms | Kangfang Pharmaceutical Co Ltd | 2022-06-29 | Microsatellite instability-high cancer; Adenocarcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Lung Neoplasms; Esophageal Squamous Cell Carcinoma; Urologic Neoplasms; Colorectal Neoplasms; Microsatellite Instability; Vulvar Neoplasms; Nasopharyngeal Carcinoma; Solid tumours; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Mismatch Repair Deficient Cancer; Carcinoma; Vaginal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Ovarian Neoplasms; Lymphoma, T-Cell, Peripheral | Details |
| Ipilimumab | 10D1; MDX-CTLA-4; Anti-CTLA-4 Mab; BMS-734016; Mab-10D14; MDX-101; MDX-010 | Approved | Bristol-Myers Squibb Company | Yervoy, 逸沃 | United States | Melanoma | Bristol-Myers Squibb Company | 2011-03-25 | Ovarian germ cell tumor; Liver Neoplasms; Leukemia, Myelogenous, Chronic; Ovarian Neoplasms; Solid tumours; Intestinal Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Skin Melanoma; Lymphoma, T-Cell, Peripheral; HIV Infections; Teratoma; Fibromatosis, Aggressive; Cystadenocarcinoma; Carcinoma, Renal Cell; Leukemia, Hairy Cell; Hemangiosarcoma; Carcinoma, Merkel Cell; Rectal Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Parathyroid Neoplasms; Pheochromocytoma; Cystadenocarcinoma, Serous; Carcinoma, Basal Cell; Cystadenocarcinoma, Mucinous; Anus Neoplasms; Stomach Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Leukemia, Myelomonocytic, Chronic; Carcinoma, Transitional Cell; Seminoma; Myelodysplastic Syndromes; Lymphoma, Large B-Cell, Diffuse; Lymphomatoid Granulomatosis; Colonic Neoplasms; Lymphoma, Large-Cell, Immunoblastic; Myeloproliferative Disorders; Carcinoma, Verrucous; Hodgkin Disease; Papillomavirus Infections; Pancreatic Neoplasms; Carcinoma, Ovarian Epithe | Details |
| Tremelimumab | CP-675206; CP-675 | Approved | Pfizer Inc | IMJUDO | United States | Carcinoma, Hepatocellular | Astrazeneca Ab | 2022-10-21 | Testicular Neoplasms; Esophageal adenocarcinoma; Gallbladder Neoplasms; Urogenital Neoplasms; Fallopian Tube Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Pinealoma; Genital Neoplasms, Female; Oropharyngeal Neoplasms; Mouth Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Bile Duct Neoplasms; Urethral Neoplasms; Vulvar Neoplasms; Sarcoma; Microsatellite Instability; Cholangiocarcinoma; Breast Neoplasms; Melanoma; Laryngeal Diseases; Adenocarcinoma; Carcinoma, Endometrioid; Dysgerminoma; Uterine Cervical Neoplasms; Hepatitis C, Chronic; Neoplasms, Germ Cell and Embryonal; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Prostatic Neoplasms; Glioma; Metastatic breast cancer; Lymphoma, T-Cell, Cutaneous; Lung Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Carcinoma, Pancreatic Ductal; Bone metastases; Cystadenoma, Serous; Vaginal Neoplasms; Digestive System Diseases; Carcinoma, Merkel Cell; Esophageal Neoplasms; Carcinoma; Stomach Neoplasms; C | Details |
| Iparomlimab/Tuvonralimab | PBS-103/PBS-105; QL-1706; PSB-205 | Approved | Qilu Pharmaceutical Co Ltd | 齐倍安 | Mainland China | Uterine Cervical Neoplasms | Qilu Pharmaceutical Co Ltd | 2024-09-26 | Colonic Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Esophageal Squamous Cell Carcinoma; Lung Neoplasms; Colorectal Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Liver Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Solid tumours; Ovarian Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CS-1002 | CS-1002; SHR-8068 | Phase 3 Clinical | Cstone Pharmaceuticals | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Bile Duct Neoplasms; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Botensilimab | AGEN-1181 | Phase 3 Clinical | Agenus Inc | Neoplasms; Uterine Cervical Neoplasms; Neoplasm Metastasis; Adenocarcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung; Endometrial Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Colorectal Neoplasms; Prostatic Neoplasms; Microsatellite Instability; Solid tumours; Colonic Neoplasms; Pancreatic Neoplasms; Fibrolamellar hepatocellular carcinoma; Esophageal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Rectal Neoplasms; Hemangiosarcoma; Liver Neoplasms; Ovarian Neoplasms | Details |
| RP-2 | RP-2 | Phase 3 Clinical | Replimune Group Inc | Neoplasms; Uveal melanoma; Colorectal Neoplasms; Precancerous Conditions | Details |
| Gotistobart | ONC-392; BNT316; BNT-316 | Phase 3 Clinical | Oncoimmune Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Hepatocellular; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Sarcoma; Prostatic Neoplasms; Carcinoma, Adenoid Cystic; Solid tumours; Salivary Gland Neoplasms; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Anorectal disorders; Cystadenocarcinoma, Serous; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Ovarian Neoplasms; Head and Neck Neoplasms | Details |
| Erfonrilimab | KN-046; KN046 | Phase 3 Clinical | Jiangsu Alphamab Biopharmaceuticals Co Ltd | Colorectal Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Esophageal Squamous Cell Carcinoma; Carcinoma, Pancreatic Ductal; Lymphoma; Thymoma; Thymus Neoplasms; Solid tumours; Breast Neoplasms; Triple Negative Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Biliary Tract Neoplasms | Details |
| Volrustomig | MEDI-5752; MEDI5752 | Phase 3 Clinical | Medimmune Llc | Biliary Tract Neoplasms; Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Mesothelioma; Sarcoma; Bile Duct Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Nurulimab/Prolgolimab | BCD-217; BCD-145/BCD-100 | Phase 3 Clinical | BIOCAD JSC | Skin Melanoma; Melanoma | Details |
| Ipilimumab biosimilar (Innovent Biologics) | IBI-310 | Phase 3 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Liver Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Mismatch Repair Deficient Cancer; Colonic Neoplasms; Neuroendocrine Tumors; Microsatellite Instability; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Pembrolizumab/Quavonlimab | MK-1308A | Phase 3 Clinical | Merck Sharp & Dohme Corp, Msd Ireland (Carlow), MSD R&D(China)Co Ltd | Solid tumours; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| YH-001 | YH-001 | Phase 2 Clinical | Eucure Pharmaceutical Technology (Beijing) Co Ltd | Solid tumours; Alopecia Areata; Sarcoma; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details |
| Danvilostomig | SI-B003 | Phase 2 Clinical | Sichuan Baili Pharmaceutical Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Neoplasm Metastasis; Melanoma | Details |
| BMS-986288 | BMS-986288 | Phase 2 Clinical | Cytomx Therapeutics Inc | Neoplasms | Details |
| Evalstotug | CAB-CTLA-4; BA-3071 | Phase 2 Clinical | Bioatla, BeOne Medicines Ltd | Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Vudalimab | XmAb-20717; XmAb-717; XmAb 717 | Phase 2 Clinical | Xencor Inc | Thymoma; Adenocarcinoma, Clear Cell; Vulvar Neoplasms; Cholangiocarcinoma; Nasopharyngeal Carcinoma; Breast Neoplasms; Colorectal Neoplasms; Astrocytoma; Castration-Sensitive Prostate Neoplasms; Carcinoma, Neuroendocrine; Prostatic Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms; Thyroid Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Hodgkin Disease; Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Carcinoma, Basal Cell; Squamous Cell Carcinoma of Head and Neck; Small Cell Lung Carcinoma; Ovarian Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Transitional Cell; Adenoma, Acidophil; Mesothelioma; Microsatellite instability-high cancer; Adnexal Diseases; Prostatic Neoplasms, Castration-Resistant | Details |
| Recombinant anti-CTLA-4 human monoclonal antibody (Hualan Biological Engineering) | Phase 2 Clinical | Hualan Genetic Engineering Co Ltd | Melanoma | Details | |
| Lorigerlimab | MGD-019; AEX1344 | Phase 2 Clinical | Macrogenics Inc | Adenocarcinoma, Clear Cell; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Uterine Cervical Neoplasms; Carcinoma, Pancreatic Ductal; Endometrial Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Ovarian Neoplasms; Prostatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Hormone-Resistant Prostate Neoplasms; Carcinoma, Ovarian Epithelial; Neoplasms; Ovarian Clear Cell Carcinoma; Carcinoma, Renal Cell; Solid tumours; Skin Melanoma | Details |
| PD-L1/CTLA4 bispecific monoclonal antibody (Changhai Hospital) | Phase 2 Clinical | Changhai Hospital Of Shanghai | Pancreatic Neoplasms | Details | |
| Abatacept (Orban Biotech) | Phase 2 Clinical | Orban Biotech, The National Institute Of Diabetes And Digestive And Kidney Diseases | Diabetes Mellitus, Type 1 | Details | |
| Quavonlimab | AK-107; MK-1308 | Phase 2 Clinical | Zhongshan Akeso Biopharma Co Ltd | Solid tumours; Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Zalifrelimab | AGEN-1884; RebmAb-600; UGN-301 | Phase 2 Clinical | 4-Antibody, Ludwig Institute For Cancer Research | Solid tumours; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Hemangiosarcoma; Pancreatic Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms; Sarcoma; Urologic Neoplasms; Carcinoma, Pancreatic Ductal; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BMS-986218 (Bristol-Myers Squibb) | BMS-986218 | Phase 2 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Prostatic Neoplasms, Castration-Resistant; Adrenal Gland Neoplasms; Prostatic Neoplasms; Lung Neoplasms | Details |
| Anti-CTLA-4 and PD-1 CAR-T cell therapy (Shanghai Cell Therapy Research Institute) | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Neoplasms | Details | |
| KD-6001 | KD-6001 | Phase 2 Clinical | Shanghai Kanda Bio-Technology Co Ltd, Shanghai Celgen Bio-Pharmaceutical Co Ltd | Solid tumours; Liver Neoplasms; Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| BT-001 | BT-001 | Phase 2 Clinical | Transgene Sa | Carcinoma, Merkel Cell; Neoplasms; Triple Negative Breast Neoplasms; Sarcoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Porustobart | HBM-4003; HCAb 4003-2 | Phase 2 Clinical | Harbour Biomed | Solid tumours; Neoplasms; Neuroendocrine Tumors; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| JK-08 | JK-08 | Phase 2 Clinical | Xinlitai (Chengdu) Biological Technology Co Ltd | Triple Negative Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Melanoma; Neoplasm Metastasis; Thyroid Neoplasms; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Pancreatic Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Carcinoma, Transitional Cell; Small Cell Lung Carcinoma; Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell | Details |
| Sovipostobart | BMS-986249 | Phase 2 Clinical | Cytomx Therapeutics Inc | Neoplasms | Details |
| GI-101 | SIM-323; SIM323; SIM0323; GI-101 | Phase 2 Clinical | GI Innovation Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Vaginal Neoplasms; Carcinoma, Merkel Cell; Carcinoma, Renal Cell; Neoplasms; Urinary Bladder Neoplasms; Vulvar Neoplasms; Sarcoma; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma | Details |
| Firastotug | ADG-116 | Phase 2 Clinical | Adagene (Suzhou) Ltd | Solid tumours | Details |
| EGL-001 | EGL-001 | Phase 2 Clinical | Egle Therapeutics | Solid tumours; Neoplasms | Details |
| COYA-302 | COYA302; COYA 302; COYA-302 | Phase 2 Clinical | Coya Therapeutics Inc | Parkinson Disease; Frontotemporal Dementia; Amyotrophic Lateral Sclerosis | Details |
| GI-102 | GI-102 | Phase 2 Clinical | GI Innovation Inc | Skin Melanoma; Ovarian Neoplasms; Solid tumours; Liver Neoplasms; Carcinoma, Renal Cell; Soft Tissue Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular; Melanoma | Details |
| Anti-CTLA-4/PD-1 expressing EGFR-CAR-T | Phase 2 Clinical | Shanghai Cell Therapy Research Institute | Solid tumours | Details | |
| Vilastobart | XTX-101; XTX101 | Phase 2 Clinical | Xilio Development Inc | Colorectal Neoplasms | Details |
| RP-3 | RP-3 | Phase 2 Clinical | Replimune Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Muzastotug | ADG-126 | Phase 2 Clinical | Adagene Inc | Solid tumours; Liver Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| PD-L1 antibody combined with CTLA-4 antibody (Shanghai Zhongshan Hospital) | Phase 2 Clinical | Shanghai Zhongshan Hospital | Cholangiocarcinoma | Details | |
| JS-007 | JS007; JS-007 | Phase 2 Clinical | Shanghai Junshi Biosciences Co Ltd | Solid tumours; Pancreatic Neoplasms; Neoplasms; Lung Neoplasms; Melanoma | Details |
| KN-044 | KN-044 | Phase 1 Clinical | Suzhou Alphamab Co Ltd | Solid tumours | Details |
| Bavunalimab | XmAb-22841; XmAb-841 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Fallopian Tube Neoplasms; Penile Neoplasms; Endometrial Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Nasopharyngeal Carcinoma; Solid tumours; Cholangiocarcinoma; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck | Details |
| ATOR-1015 | ADC-1015; ATOR-1015 | Phase 1 Clinical | Alligator Bioscience Ab | Solid tumours; Neoplasms | Details |
| Anti-CTLA-4 monoclonal antibody (Regeneron) | REGN-4659 | Phase 1 Clinical | Regeneron Pharmaceuticals Inc | Carcinoma, Non-Small-Cell Lung | Details |
| Ipilimumab biosimilar (Henlius) | HLX13 | Phase 1 Clinical | Shanghai Henlius Biotech Inc | Liver Neoplasms; Solid tumours; Esophageal Neoplasms; Carcinoma, Renal Cell; Mesothelioma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Ipilimumab biosimilar (Mab-Venture/ShuangLu Pharmaceutical) | MV-049 | Phase 1 Clinical | Beijing Sl Pharmaceutical Co Ltd, Mab-Venture Biopharm Co Ltd | Solid tumours | Details |
| VDJ-142 | VDJ-142 | Phase 1 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid; Lupus Erythematosus, Systemic; Inflammation | Details |
| SAL-008 | SAL-008 | Phase 1 Clinical | Xinlitai (Chengdu) Biological Technology Co Ltd | Neoplasms | Details |
| GIGA-564 | GIGA-564 | Phase 1 Clinical | GigaGen Inc | Solid tumours; Neoplasms | Details |
| CTLA-4 Mab (Beijng Immunoah Pharma Tech) | 5E3 (Beijng Immunoah Pharma Tech); CTLA-4 Mab (Beijng Immunoah Pharma Tech) | Phase 1 Clinical | Beijng Immunoah Pharma Tech Co Ltd | Solid tumours | Details |
| CS-2009 | CS-2009 | Phase 1 Clinical | Cstone Pharmaceuticals | Solid tumours; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| A-337 (Klus Pharma) | A337 (Klus Pharma); A-337 (Klus Pharma) | Phase 1 Clinical | Klus Pharma Inc | Neoplasms | Details |
| BZE-2209 | BZE-2209; BZE2209; αPD1/CTLA4-MSLN-CAR T Cells | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Solid tumours | Details |
| HC-010(Shanghai Hongcheng) | HC-010; HC010 | Phase 1 Clinical | Shanghai Hongcheng Pharmaceutical Co Ltd | Solid tumours | Details |
| STW204/Gotistobart | AI-061 | Phase 1 Clinical | Oncoc4 Inc | Colorectal Neoplasms; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Endometrial Neoplasms; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Bile Duct Neoplasms; Ovarian Neoplasms; Urinary Bladder Neoplasms; Squamous Cell Carcinoma of Head and Neck; Cystadenocarcinoma, Serous; Carcinoma, Renal Cell; Stomach Neoplasms; Anus Neoplasms; Esophageal Neoplasms | Details |
| KM602 | KM602; KM-602; XZP-KM602 | Phase 1 Clinical | Xuanzhu (Shijiazhuang) Biotechnology Co Ltd | Solid tumours | Details |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| SV-102 | SV-102; SV102; SYNC-T SV-102 | Phase 1 Clinical | Syncromune Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| IMM-27M | IMM-27M; IMM27M; SYN27M; SYN-27M | Phase 1 Clinical | ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd | Solid tumours; Neoplasms; Pathologic Processes; Carcinoma, Hepatocellular | Details |
| Imiquimod/Zalifrelimab | UGN-302; UGN-201+UGN-301 | Phase 1 Clinical | Urogen Pharma | Urinary Bladder Neoplasms | Details |
| TWP-102 | TWP-102 | Phase 1 Clinical | Therawisdom Biopharma Co Ltd | Neoplasms | Details |
| SKB-337 | SKB337; A-337; SKB-337 | Phase 1 Clinical | Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Solid tumours | Details |
| BAT-4706 | BAT-4706 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Melanoma | Details |
| B7-2/GM-CSF cancer gene therapy | CIT | Phase 1 Clinical | Radient | Neoplasms | Details |
This web search service is supported by Google Inc.







