
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| GIR-H52D3 | Human | Human GIPR Protein, His,Flag Tag (Detergent) |  |
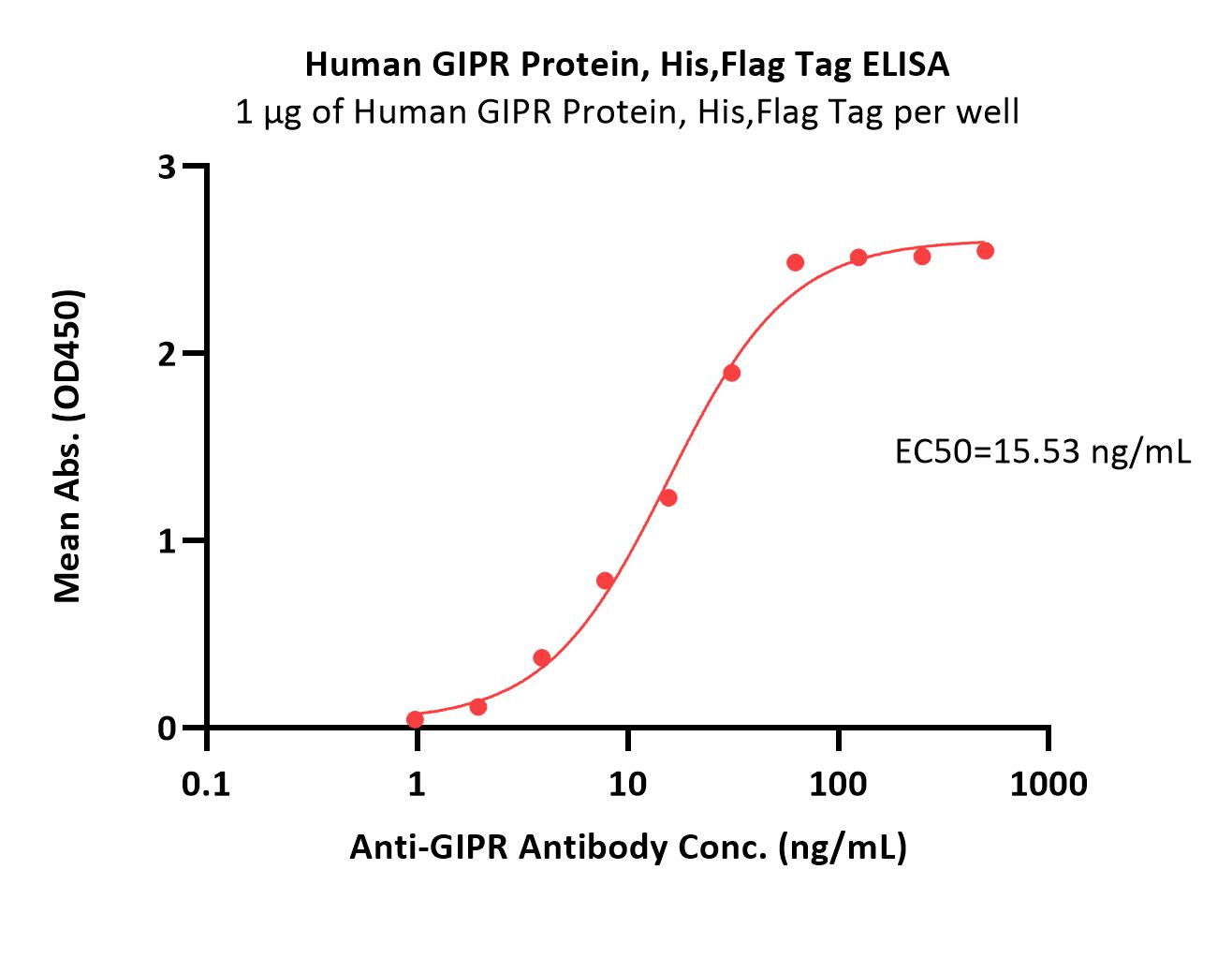
|
|
| CHEK-ATP208 | Human | HEK293/Human GIPR Stable Cell Line (Low Expression) | |||
| CHEK-ATP207 | Human | HEK293/Human GIPR Stable Cell Line (Medium Expression) | |||
| CHEK-ATP206 | Human | HEK293/Human GIPR Stable Cell Line (High Expression) | |||
| GIR-H82E3 | Human | Biotinylated Human GIPR Protein, His,Avitag™ (MALS verified) |  |
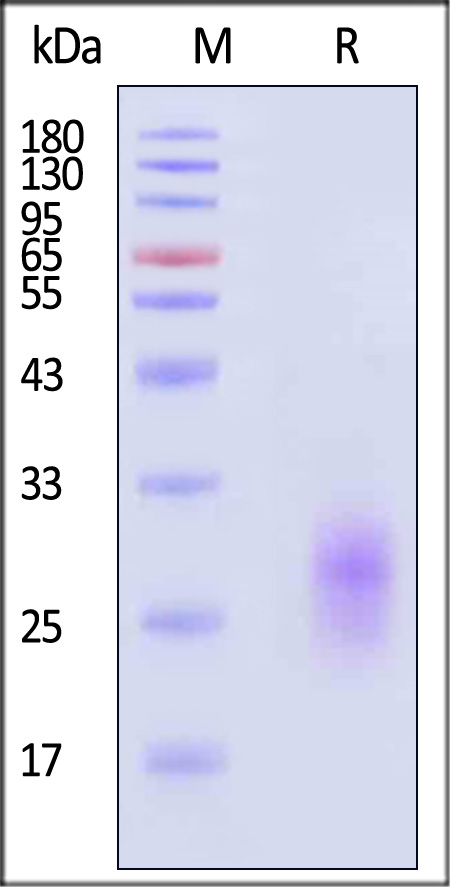
|
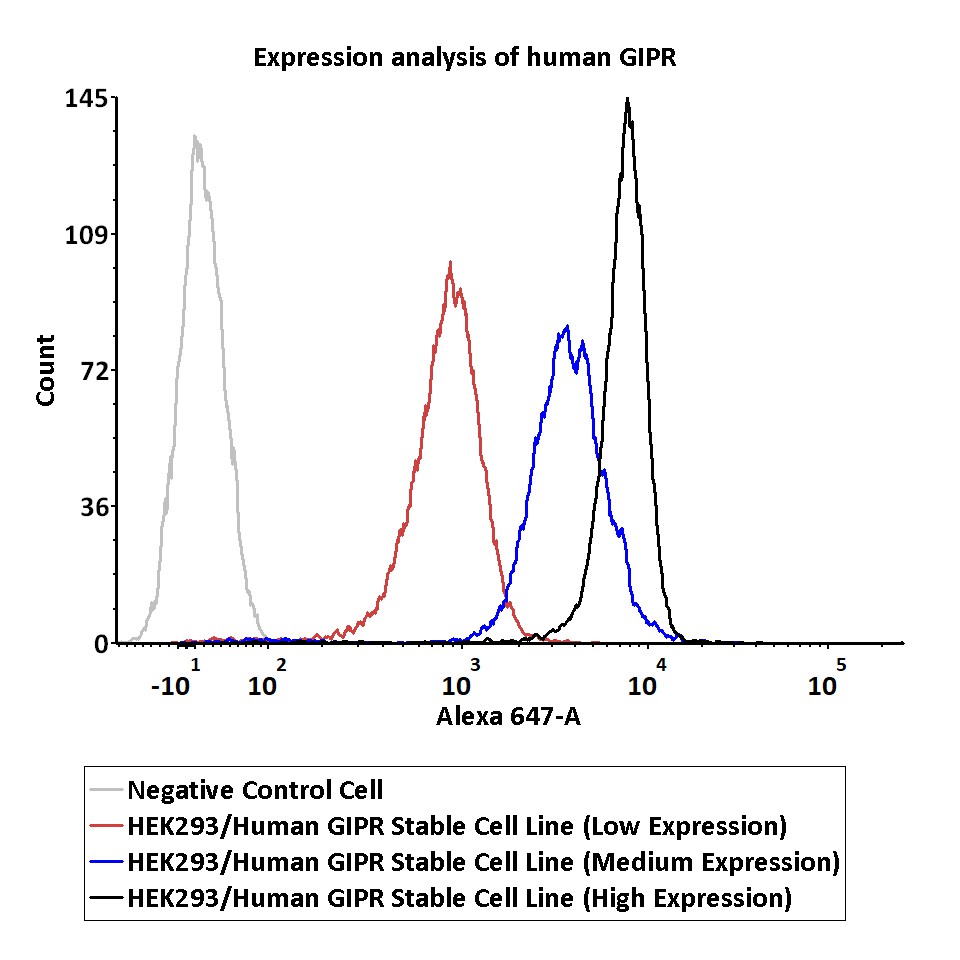
Expression analysis of human GIPR on HEK293/Human GIPR Stable Cell Line by FACS.
Cell surface staining using Alexa 647-labeled anti-human GIPR antibody was performed on HEK293/Human GIPR Stable Cell Line with different expression levels: HEK293/Human GIPR Stable Cell Line (Low Expression); HEK293/Human GIPR Stable Cell Line (Medium Expression); HEK293/Human GIPR Stable Cell Line (High Expression).
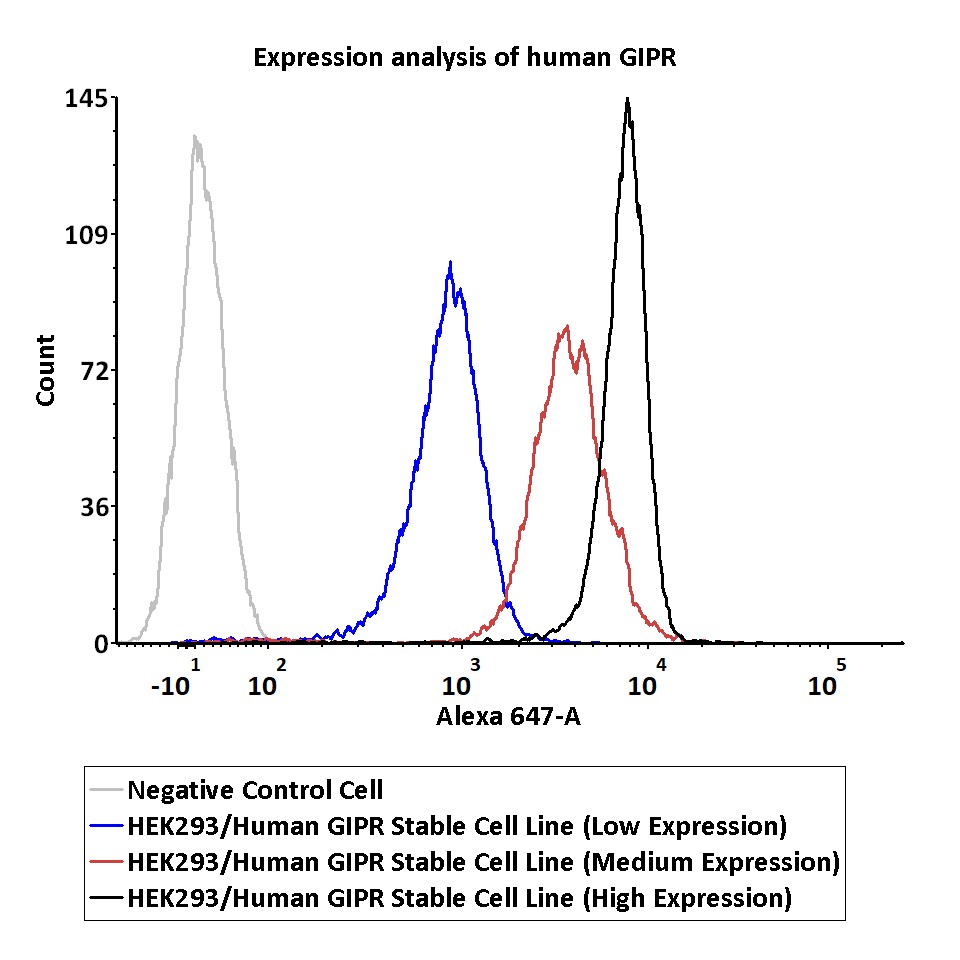
Expression analysis of human GIPR on HEK293/Human GIPR Stable Cell Line by FACS.
Cell surface staining using Alexa 647-labeled anti-human GIPR antibody was performed on HEK293/Human GIPR Stable Cell Line with different expression levels: HEK293/Human GIPR Stable Cell Line (Low Expression); HEK293/Human GIPR Stable Cell Line (Medium Expression); HEK293/Human GIPR Stable Cell Line (High Expression).

The purity of Biotinylated Human GIPR Protein, His,Avitag (Cat. No. GIR-H82E3) is more than 85% and the molecular weight of this protein is around 20-30 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Tirzepatide | LY-3298176; GIP/GLP-1 RA | Approved | Eli Lilly And Company | Mounjaro, Zepbound, 穆峰达 | United States | Diabetes Mellitus, Type 2 | Eli Lilly And Company | 2022-05-13 | Metabolic Diseases; Nutritional and Metabolic Diseases; Kidney Failure, Chronic; Diabetes Mellitus; Overweight; Renal Insufficiency, Chronic; Obesity; Alcoholism; Glucose Metabolism Disorders; Hepatic Insufficiency; Cardiovascular Diseases; Breast Neoplasms; Prostatic Neoplasms; Substance-Related Disorders; Sleep Apnea Syndromes; Endocrine System Diseases; Obesity, Morbid; Hypoglycemia; Opioid-Related Disorders; Sleep Apnea, Obstructive; Weight Gain; Metabolic Dysfunction-Associated Steatotic Liver Disease; Renal Insufficiency; Heart Failure; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 1 | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Retatrutide | LY-3437943 | Phase 3 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 2; Atherosclerosis; Renal Insufficiency; Sleep Apnea, Obstructive; Hepatic Insufficiency; Osteoarthritis, Knee; Cardiovascular Diseases; Renal Insufficiency, Chronic; Obesity; Overweight | Details |
| BGM-0504 | BGM-0504 | Phase 3 Clinical | Brightgene Bio-Medical Technology Co Ltd | Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Obesity; Overweight | Details |
| HRS-9531 | HRS-9531; HRS9531 | Phase 3 Clinical | Fujian Suncadia Medicine Co Ltd | Heart Failure, Diastolic; Diabetes Mellitus, Type 2; Polycystic Ovary Syndrome; Body Weight; Obesity; Overweight; Diabetes Mellitus | Details |
| HS-20094 | HS-20094 | Phase 3 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| CT-868 | CT-868; RG-6641 | Phase 2 Clinical | Carmot Therapeutics Inc | Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| Efocipegtrutide | HM-15211 | Phase 2 Clinical | Hanmi Pharmaceutical Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Obesity | Details |
| NNC0480-0389 | NNC0480-0389 | Phase 2 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| HDM-1005 | HDM-1005; HDM1005 | Phase 2 Clinical | Hangzhou Zhongmei Huadong Pharmaceutical Co Ltd | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| PF-07976016 | PF-07976016 | Phase 2 Clinical | Pfizer Inc | Obesity | Details |
| NNC0519-0130 | NNC0519-0130 | Phase 2 Clinical | Novo Nordisk A/S | Diabetes Mellitus, Type 2; Obesity; Renal Insufficiency, Chronic | Details |
| MWN-101 | MWN-101 | Phase 2 Clinical | Shanghai Minwei Biotechnology Co Ltd | Diabetes Mellitus, Type 2; Sleep Apnea, Obstructive; Obesity; Overweight | Details |
| RAY-1225 | RAY1225; RAY-1225 | Phase 2 Clinical | Guangdong Raynovent Biotech Co Ltd | Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Acute Kidney Injury; Hepatic Insufficiency; Obesity; Overweight | Details |
| VK-2735 | VK-2735 | Phase 2 Clinical | Viking Therapeutics Inc | Weight Loss; Metabolic Dysfunction-Associated Steatotic Liver Disease; Obesity | Details |
| THDBH-120 | THDBH120; THDBH121 | Phase 2 Clinical | Wuxi Apptec(Shanghai) Co Ltd | Diabetes Mellitus, Type 2; Obesity; Overweight; Diabetes Mellitus | Details |
| CT-388 | CT-388; RG-6640 | Phase 2 Clinical | Carmot Therapeutics Inc | Diabetes Mellitus, Type 2; Obesity; Overweight; Diabetes Mellitus | Details |
| Maridebart cafraglutide | AMG-133; AMG133 | Phase 2 Clinical | Amgen Inc | Diabetes Mellitus, Type 2; Body Weight; Obesity; Overweight | Details |
| LY-3493269 | LY-3493269 | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 2 | Details |
| SCO-094 | SCO-094; tak-094 | Phase 1 Clinical | Takeda | Diabetes Mellitus, Type 2 | Details |
| NN9542 | NN9542; NN-9542 | Phase 1 Clinical | Novo Nordisk A/S | Obesity | Details |
| Gastric inhibitory polypeptide receptor agonist long acting II therapeutic (Eli Lilly and Company) | Phase 1 Clinical | Eli Lilly And Company | Obesity; Diabetes Mellitus | Details | |
| NNC0650-0013 | NNC0650-0013; NN9650; NN-9650 | Phase 1 Clinical | Novo Nordisk A/S | Details | |
| ZX-2010 | ZX2010; ZX-2010 | Phase 1 Clinical | Jiangsu Zhongxin Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| MWN-105 | MWN-105 | Phase 1 Clinical | Shanghai Minwei Biotechnology Co Ltd | Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Obesity; Overweight; Hyperlipidemias | Details |
| ZX-2021 | ZX2021; ZX-2021 | Phase 1 Clinical | Jiangsu Zhongxin Pharmaceutical Co Ltd | Diabetes Mellitus, Type 2; Obesity; Overweight | Details |
| HZ-012 | DR-10628; HZ-012 | Phase 1 Clinical | Hangzhou Heze Pharmaceutical Technology Co Ltd, Zhejiang Doer Biologics Corp | Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus, Type 2; Obesity; Diabetes Mellitus; Overweight | Details |
| DR-10627 | HZ-010; DR-10627 | Phase 1 Clinical | Hangzhou Heze Pharmaceutical Technology Co Ltd, Zhejiang Doer Biologics Corp | Diabetes Mellitus, Type 2; Metabolic Dysfunction-Associated Steatotic Liver Disease; Diabetes Mellitus | Details |
| Macupatide | LY-3532226 | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 1; Obesity | Details |
| LY-3537031 | LY3537031; LY-3537031 | Phase 1 Clinical | Eli Lilly And Company | Obesity; Diabetes Mellitus | Details |
| LY-3537021 | LY-3537021 | Phase 1 Clinical | Eli Lilly And Company | Diabetes Mellitus, Type 2 | Details |
| GMA-106 | GMA106; GMA-106 | Phase 1 Clinical | Gmax Biopharm Llc | Obesity; Overweight | Details |
| GIP[3-30]NH2 | GIP[3-30]NH2 | Copenhagen University Hospital Gentofte, University Of Copenhagen | Details |
This web search service is supported by Google Inc.







