
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| FRα BsAb - 01 | PCC | Solid Tumor | Ovarian cancer,Non-small cell lung cancer |
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| FRα BsAb-ADC | Bispecific antibody | Oncology/Cancer | Ovarian cancer | Preclinical | Global |

Expression analysis of human FOLR1 on HEK293/Human FOLR1 Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human FOLR1 Stable Cell Line or negative control cell using PE-labeled anti-human FOLR1 antibody.
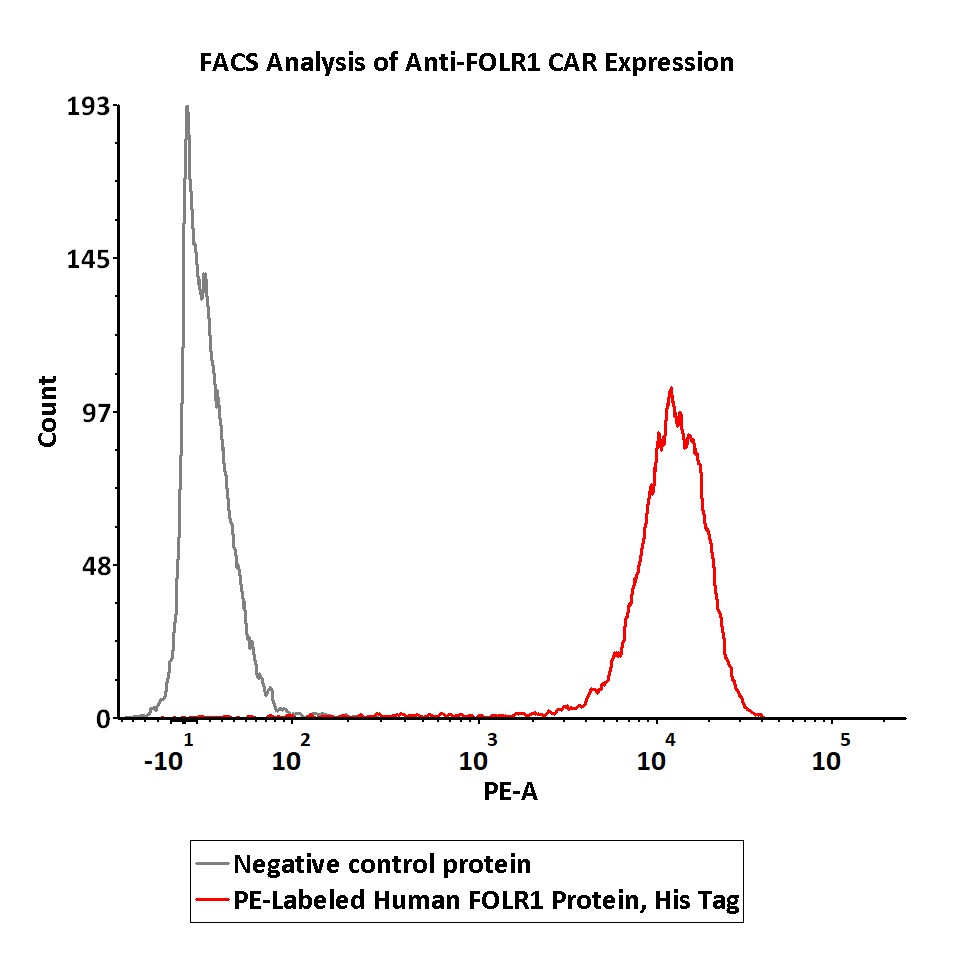
5e5 of anti-FOLR1 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human FOLR1, His Tag (Cat. No. FO1-HP2H9) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
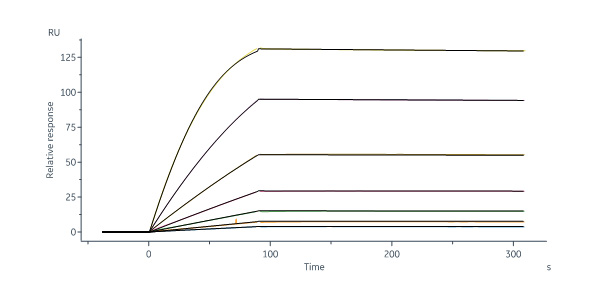
Biotinylated Human FOLR1, His,Avitag (Cat. No. FO1-H82E2) immobilized on SA Chip can bind Folic acid-BSA with an affinity constant of 83.8 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Pafolacianine Sodium | Pteroyl-L-Tyr-S0456; OTL-38; EC-17; OTL-0038 | Approved | On Target Laboratories Inc | Cytalux | United States | Ovarian Neoplasms | On Target Laboratories Inc | 2021-11-29 | Ovarian Neoplasms; Inflammatory Bowel Diseases; Neoplasms; Arthritis, Rheumatoid; Breast Neoplasms; Pituitary Neoplasms; Peritoneal Neoplasms; Lung Neoplasms; Gastrointestinal Neoplasms | Details |
| Mirvetuximab soravtansine | TAK-853; M9346-Asulfo-SPDB-DM4; IMGN-853; M9346A-sSPDB-DM4 | Approved | Immunogen Inc | Elahere | United States | Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial | Immunogen Inc | 2022-11-14 | Ovarian Neoplasms; Solid tumours; Fallopian Tube Diseases; Cystadenoma, Serous; Carcinoma, Ovarian Epithelial; Neoplasms; Triple Negative Breast Neoplasms; Breast Neoplasms; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinosarcoma; Uterine Neoplasms; Peritoneal Diseases | Details |
| Folinic Acid | FTHF | Approved | Autism Spectrum Disorder; Anemia; Down Syndrome; Rectal Neoplasms; Diarrhea; Colorectal Neoplasms; Vitamin B 12 Deficiency | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CBP-1008 | CBP-1008 | Phase 3 Clinical | Coherent Biopharma Suzhou Co Ltd | Ovarian Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Carcinoma, Squamous Cell; Fallopian Tube Neoplasms | Details |
| Arfolitixorin | ISO-901; 6R-MTHF | Phase 3 Clinical | Isofol Medical Ab | Rectal Neoplasms; Colonic Neoplasms; Osteosarcoma; Colorectal Neoplasms | Details |
| Luveltamab tazevibulin | STRO-002 | Phase 3 Clinical | Sutro Biopharma Inc | Solid tumours; Ovarian Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Endometrioid; Carcinoma, Non-Small-Cell Lung | Details |
| Rinatabart Sesutecan | GEN1184; GEN-1184; PRO-1184 | Phase 3 Clinical | ProfoundBio (Suzhou) Co Ltd | Ovarian Neoplasms; Solid tumours; Cystadenocarcinoma, Serous; Triple Negative Breast Neoplasms; Mesothelioma; Breast Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Farletuzumab | MORAb-003 | Phase 2 Clinical | Morphotek Inc | Ovarian Neoplasms; Solid tumours; Adenocarcinoma of Lung; Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| AZD5335 | AZD5335; AZD-5335 | Phase 2 Clinical | Astrazeneca Plc | Ovarian Neoplasms; Solid tumours; Adenocarcinoma of Lung | Details |
| Farletuzumab ecteribulin | MORAb-202 | Phase 2 Clinical | Morphotek Inc, Bristol-Myers Squibb Company, Eisai Co Ltd | Solid tumours; Ovarian Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BAT-8006 | BAT8006; BAT-8006 | Phase 2 Clinical | Bio-Thera Solutions Ltd | Solid tumours; Ovarian Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| 4S-CAR-FRa | 4S-CAR-FRa | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Carcinoma, Transitional Cell; Urinary Bladder Neoplasms | Details |
| CBP-1019 | CBP-1019 | Phase 2 Clinical | Coherent Biopharma Suzhou Co Ltd | Solid tumours; Ovarian Neoplasms; Esophageal Neoplasms; Thoracic Neoplasms; Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Urogenital Neoplasms; Lung Neoplasms; Gastrointestinal Neoplasms | Details |
| Noscapine-folate conjugate | Phase 1 Clinical | Emory University | Hematologic Neoplasms; Inflammation | Details | |
| MOv-18 IgE | MOv-18 IgE; MOv18; MOv-18 | Phase 1 Clinical | King'S College London, Cancer Research UK | Ovarian Neoplasms; Neoplasms | Details |
| ITIL-306 | ITIL-306 | Phase 1 Clinical | Instil Bio Inc | Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| IMGN-151 | IMGN-151 | Phase 1 Clinical | Immunogen Inc | Ovarian Neoplasms; Cystadenocarcinoma, Serous; Breast Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Details |
| FH-FOLR1 Chimeric Antigen Receptor T Cell Therapy(Fred Hutchinson Cancer Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center | Leukemia, Myeloid, Acute | Details | |
| LY-4170156 | LY4170156; LY-4170156 | Phase 1 Clinical | Eli Lilly And Company, Loxo Oncology Inc | Ovarian Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Endometrial Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ZW-191 | ZW-191 | Phase 1 Clinical | Zymeworks Inc | Solid tumours; Ovarian Neoplasms; Genital Neoplasms, Female; Carcinoma, Non-Small-Cell Lung | Details |
| AMT-151 | AMT-151 | Phase 1 Clinical | Multitude Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Adenocarcinoma of Lung; Neoplasms; Mesothelioma; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Endometrioid | Details |
| UB-TT170 | UB-TT170; UB-TT-170; UB_TT170; UB_TT-170 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| CBP-1018 | CBP-1018 | Phase 1 Clinical | Coherent Biopharma Suzhou Co Ltd | Solid tumours; Carcinoma, Renal Cell; Prostatic Neoplasms; Lung Neoplasms | Details |
This web search service is supported by Google Inc.







