
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| GMP-GMFH28 | Human | GMP Human GM-CSF Protein | 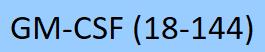 |

|
|
| CRS-B007 | Human | ClinMax™ Human GM-CSF ELISA Kit | |||
| GMF-M5211 | Mouse | Mouse GM-CSF Protein, Tag Free |  |

|
|
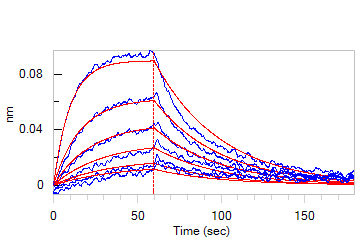
| GMF-H8214 | Human | Biotinylated Human GM-CSF Protein, epitope tag free, ultra sensitivity, primary amine labeling |  |
|
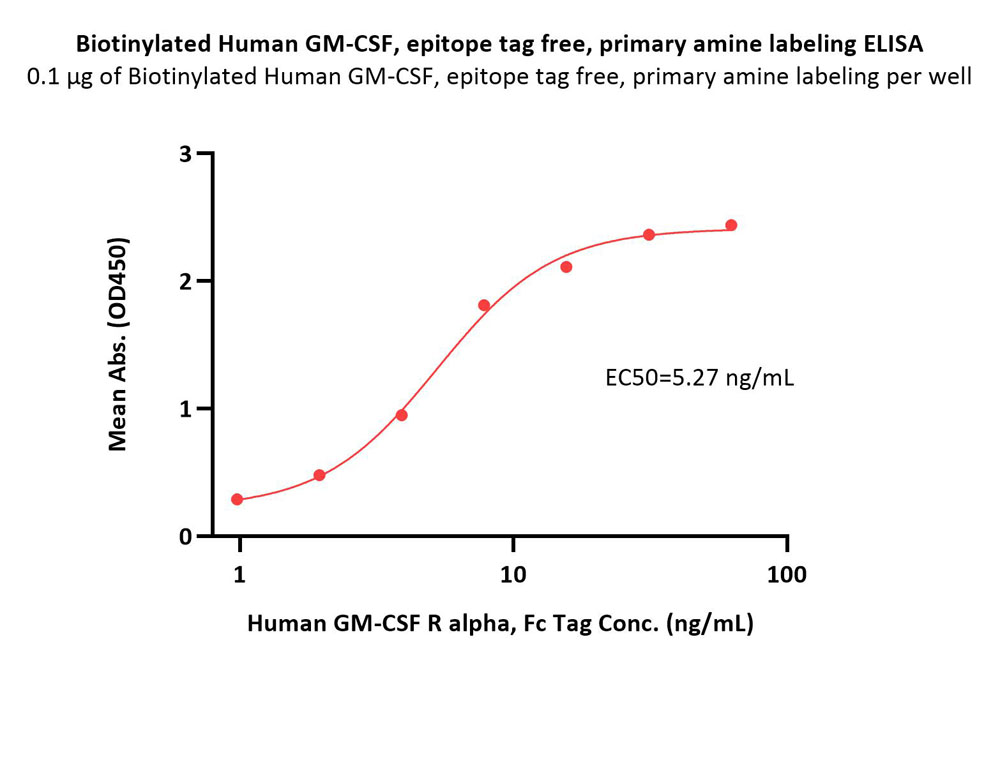
|
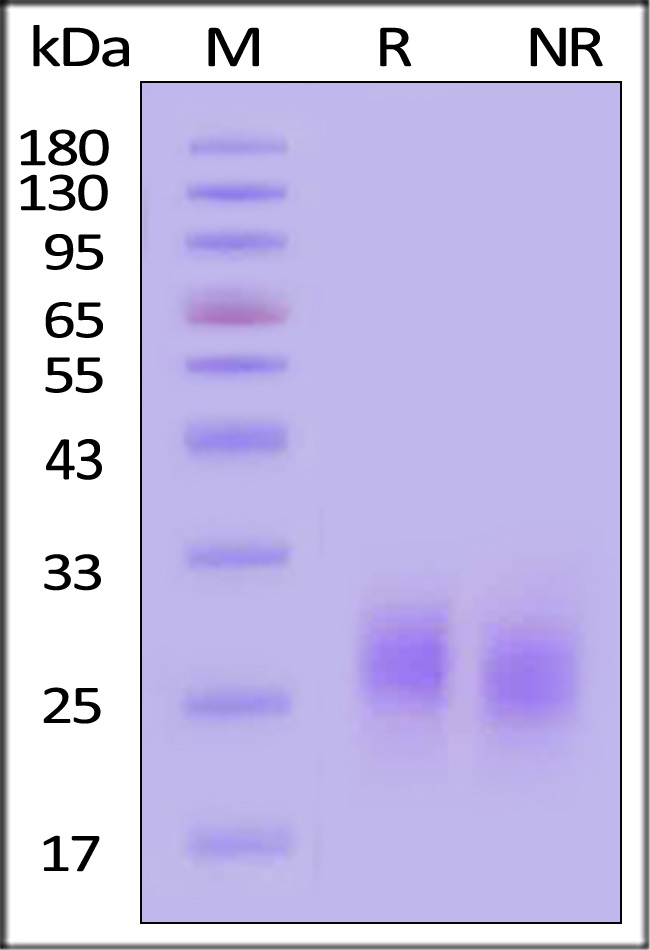
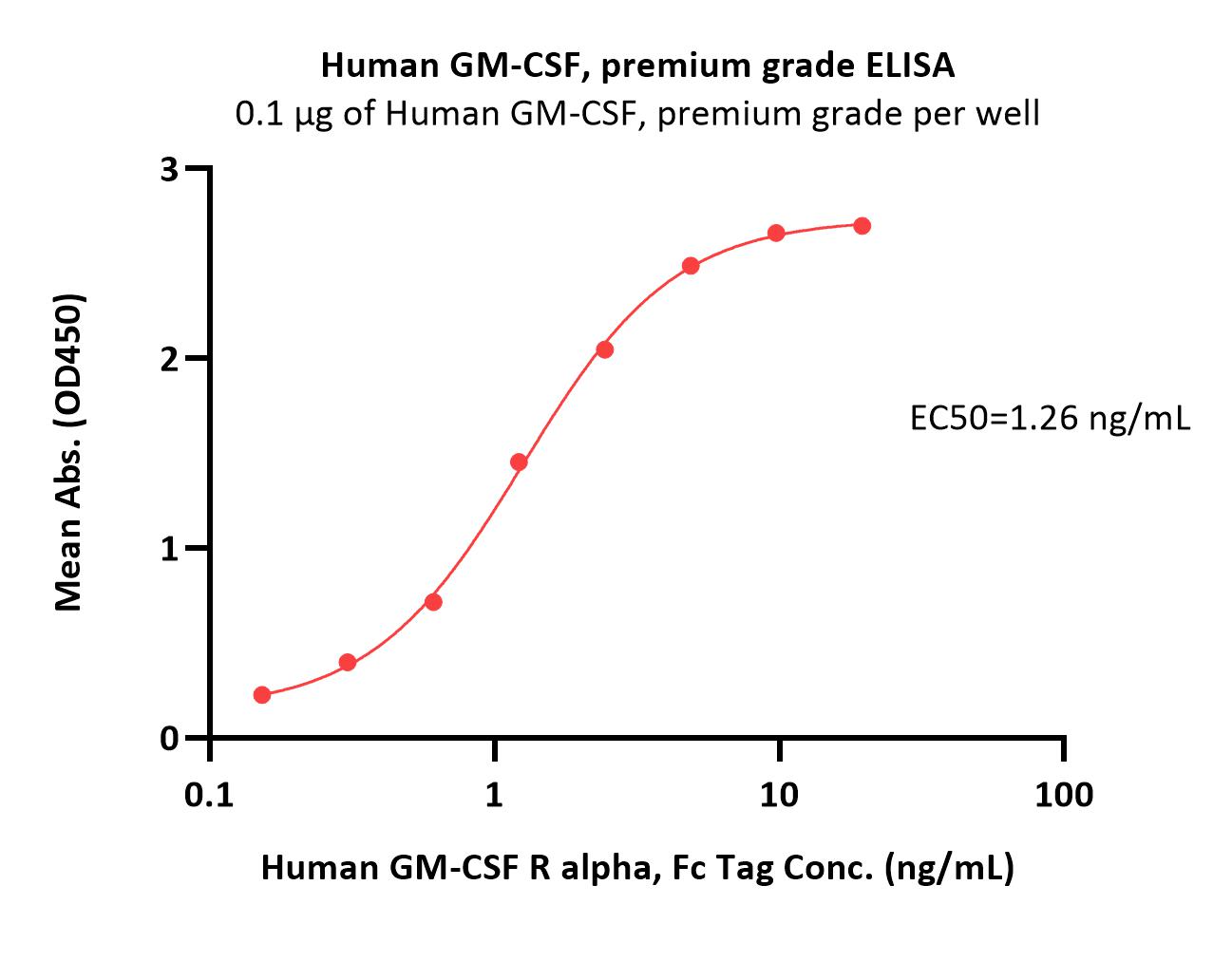
| GMF-H4214 | Human | Human GM-CSF Protein, premium grade |  |
|
|

GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) stimulates proliferation of TF-1 cells. The specific activity of GMP Human GM-CSF Protein is > 5.00ⅹ10^6 IU/mg, which is calibrated against human GM-CSF WHO International Standard (NIBSC code: 88/646) (QC tested).

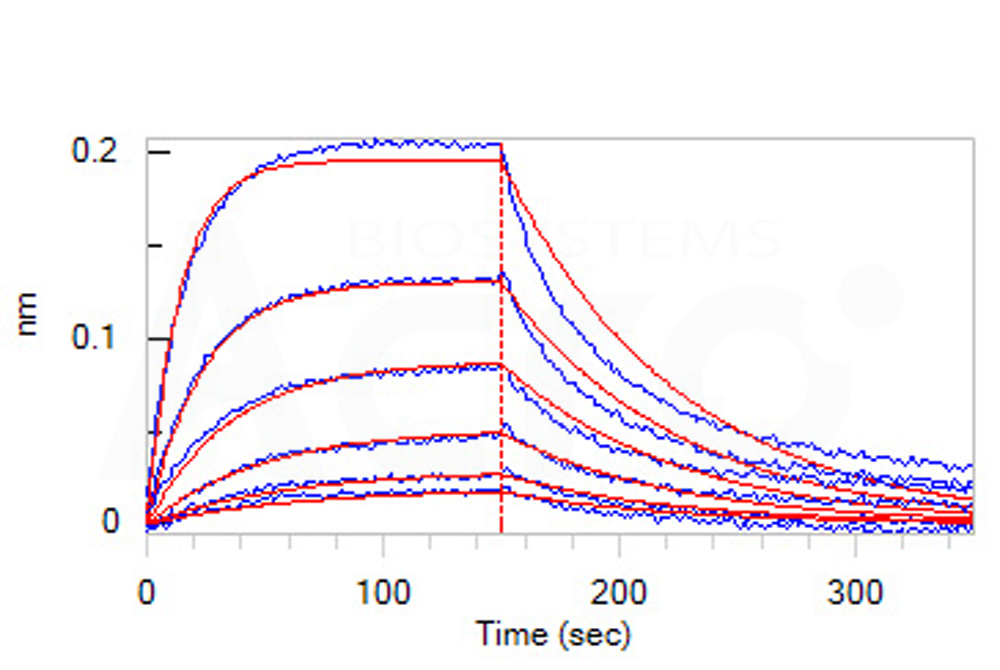
Loaded Biotinylated Human GM-CSF, epitope tag free, primary amine labeling (Cat. No. GMF-H8214) on SA Biosensor, can bind Human GM-CSF R alpha, His Tag (SPR verified) (Cat. No. GRA-H52H7) with an affinity constant of 23.1 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Otilimab | MOR-04357; GSK-3196165; GSK-165; MOR-103 | Phase 3 Clinical | University Of Melbourne | Stomach Neoplasms; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Spondylarthritis; Multiple Sclerosis; Severe Acute Respiratory Syndrome; Osteoarthritis | Details |
| Plonmarlimab | TJM-2; TJ-003234; TJ-003234RAR101 | Phase 3 Clinical | I-Mab Biopharma Co Ltd | Macrophage Activation Syndrome; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Arthritis, Gouty; Lymphohistiocytosis, Hemophagocytic | Details |
| Pexastimogene devacirepvec | TG-6006; JX-594 | Phase 3 Clinical | Sillajen Inc, Regeneron Pharmaceuticals Inc, National Cancer Institute, Samsung Medical Center & Samsung Electronics Devices Llc | Sarcoma; Neoplasm Metastasis; Melanoma; Carcinoma, Hepatocellular; Lymphoma; Lung Neoplasms; Colorectal Neoplasms; Sarcoma, Ewing; Neuroblastoma; Solid tumours; Breast Neoplasms; Wilms Tumor; Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Rhabdomyosarcoma; Liver Neoplasms; Ovarian Neoplasms | Details |
| Gemogenovatucel-T | IND-14205 | Phase 3 Clinical | Gradalis Inc | Liver Neoplasms; Ovarian Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Sarcoma, Ewing; Breast Neoplasms; Peritoneal Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Namilumab | MT-203; AMG-203; IZN-101 | Phase 2 Clinical | Takeda Pharmaceutical Co Ltd, Amgen Inc | Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid; Sarcoidosis; Sarcoidosis, Pulmonary; Plaque psoriasis | Details |
| PBI-1402 | PBI-1402 | Phase 2 Clinical | Prometic Life Sciences | Anemia | Details |
| BT-001 | BT-001 | Phase 2 Clinical | Transgene Sa | Carcinoma, Merkel Cell; Neoplasms; Triple Negative Breast Neoplasms; Sarcoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Melanoma | Details |
| Breast cancer therapy (BriaCell Therapeutics) | Phase 2 Clinical | Briacell Therapeutics Corp | Breast Neoplasms; Metastatic breast cancer | Details | |
| Gimsilumab | MORAb-022; KIN-1901 | Phase 2 Clinical | Morphotek Inc | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Rheumatic Diseases; Inflammation | Details |
| MEDI-5395 | MEDI-5395; MEDI5395 | Phase 1 Clinical | Medimmune | Solid tumours | Details |
This web search service is supported by Google Inc.







