
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| BHE-H52H3 | Human | Human BCHE / Butyrylcholinesterase Protein, His Tag (active enzyme) |  |
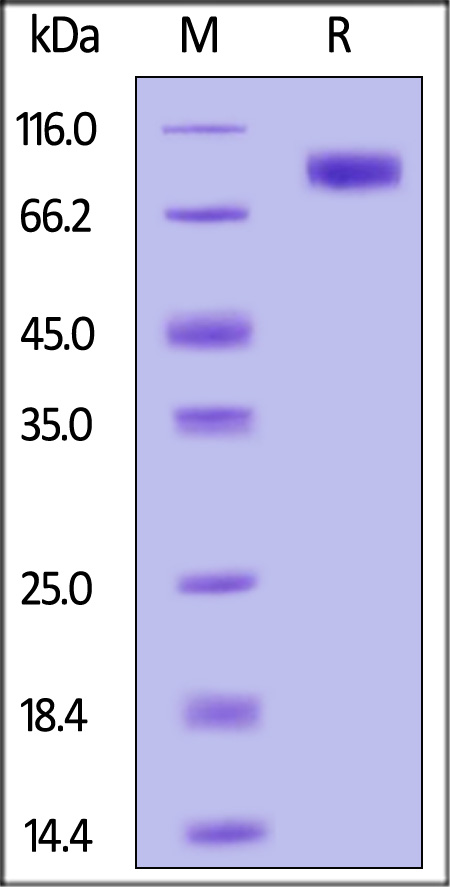
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Rivastigmine Tartrate | SDZ-212-713; SDZ 212713; SDZ-ENA-713; ONO-2540; ENA-713; ENA-713D | Approved | Novartis Pharma Ag | Exelon TDS, Rivastach, Exelon MR, Prometax, Exelon, 艾斯能 | EU | Parkinson Disease; Dementia; Alzheimer Disease | Novartis Europharm Ltd | 1998-05-11 | Substance-Related Disorders; Brain Injuries, Traumatic; Down Syndrome; Delirium; Neurobehavioral Manifestations; Alzheimer Disease; Dementia; Parkinson Disease; Cocaine-Related Disorders; Dementia, Vascular; Cognition Disorders; Anticholinergic Syndrome | Details |
| Rivastigmine transdermal patch (Luye Pharma) | LY-03013; LY-30410; RID-TDS | Approved | Acino International AG | Kingsmin, Rivirec | Mainland China | Alzheimer Disease | Luye Pharma Group Ltd | Alzheimer Disease; Parkinson Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Bisnorcymserine | ANVS-301 | Phase 1 Clinical | Horizon Therapeutics PLC | Alzheimer Disease | Details |
This web search service is supported by Google Inc.







