
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| MME-H521b | Human | Human Neprilysin / MME / CD10 Protein, Tag Free (active enzyme) |  |

|
|
| MME-H526a | Human | Human Neprilysin / MME / CD10 Protein, Fc Tag |  |
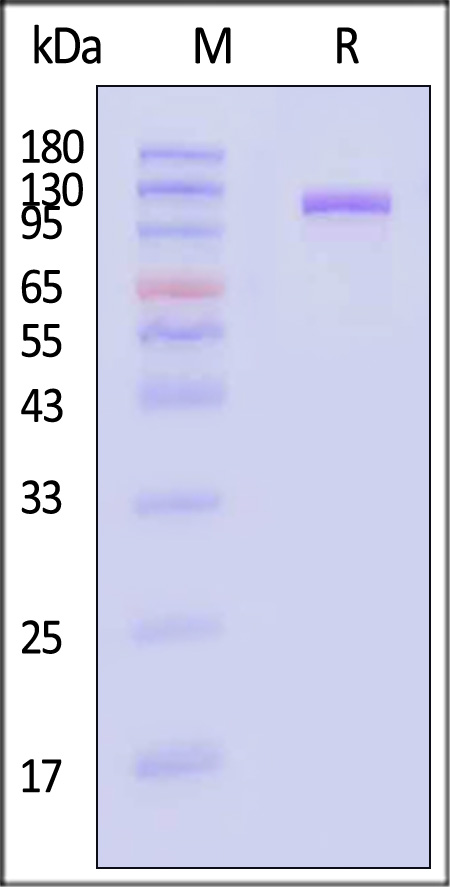
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Sacubitril/Valsartan Sodium Hydrate | LCZ-696; LCZ-696A | Approved | Novartis Pharma Ag | Enrest, Entresto, 诺欣妥, 科畅欣, Neparvis, ENTRESTO SPRINKLE, Enresuto | United States | Heart Failure | Novartis Pharmaceuticals Corp | 2015-07-07 | Breast Neoplasms; Tetralogy of Fallot; Ventricular Dysfunction, Left; Heart Failure, Systolic; Peripheral Arterial Disease; Obesity; Ebstein Anomaly; Lymphoma; Cardiovascular Diseases; Hepatic Insufficiency; Essential Hypertension; Erectile Dysfunction; Hypertension; Coronavirus Disease 2019 (COVID-19); Postmyocardial Infarction Syndrome; Heart Diseases; Cardiomyopathy, Hypertrophic; Myocardial Infarction; Renal Insufficiency; Heart Failure; Heart Failure, Diastolic | Details |
| Racecadotril | Tiorfan; Hidrasec | Approved | Bioprojet Pharma | France | Diarrhea | Bioprojet Pharma | 1993-03-23 | Diarrhea, Infantile; Diarrhea | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Dexecadotril | Phase 3 Clinical | Bioprojet Pharma | Gastrointestinal Diseases | Details | |
| Sacubitril/Valsartan(Chong Kun Dang Pharmaceutical) | CKD-202A | Phase 3 Clinical | Chong Kun Dang Pharmaceutical Corp | Essential Hypertension | Details |
| QR-12000 | QR-12000 | Phase 3 Clinical | Wuhan Createrna Science and Technology Co Ltd | Hypertension; Essential Hypertension | Details |
| Debio-0827 | PL-37; Debio-0827 | Phase 2 Clinical | Pharmaleads | Pain | Details |
| Sacubitril | AHU-377 | Phase 2 Clinical | Novartis Pharma Ag | Heart Failure; Cardiomyopathy, Hypertrophic; Insulin Resistance; Hypertension; Metabolic Diseases; Essential Hypertension; Cardiovascular Diseases; Diabetes Mellitus | Details |
| Anti-CD10 CAR T-cell therapy (Zhujiang Hospital/Nanfang Hospital) | Phase 1 Clinical | Southern Medical University Zhujing Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
This web search service is supported by Google Inc.







