
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| SOT-H8245 | Human | Biotinylated Human SOST / Sclerostin Protein, His Tag, ultra sensitivity (primary amine labeling) |  |
|
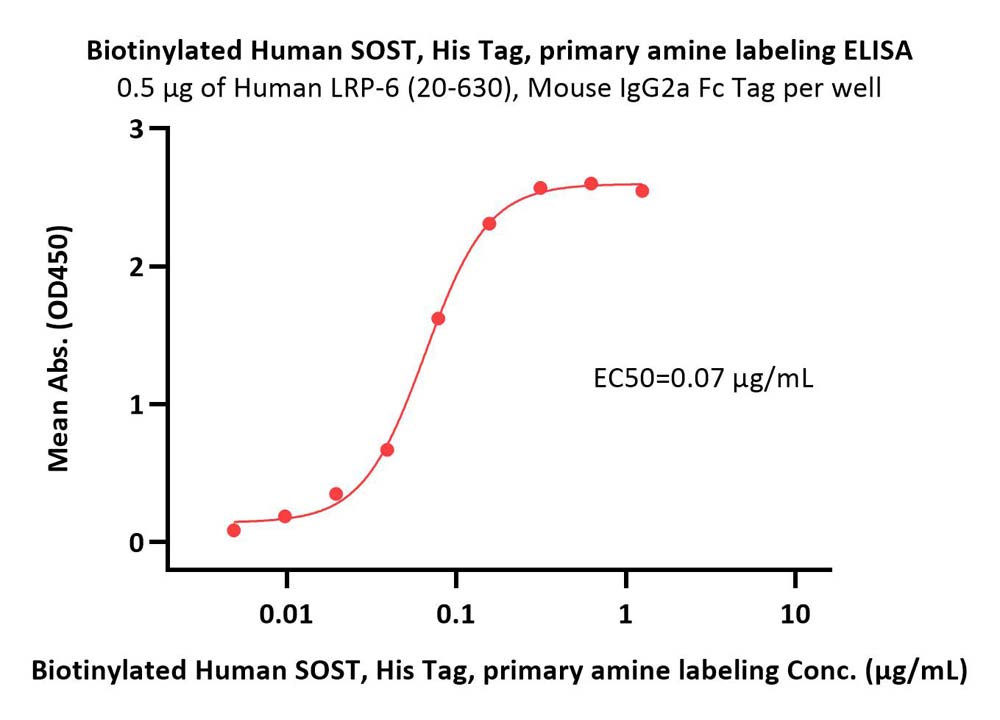
|
| HST-H5245 | Human | Human SOST / Sclerostin Protein, His Tag (MALS verified) |  |
|
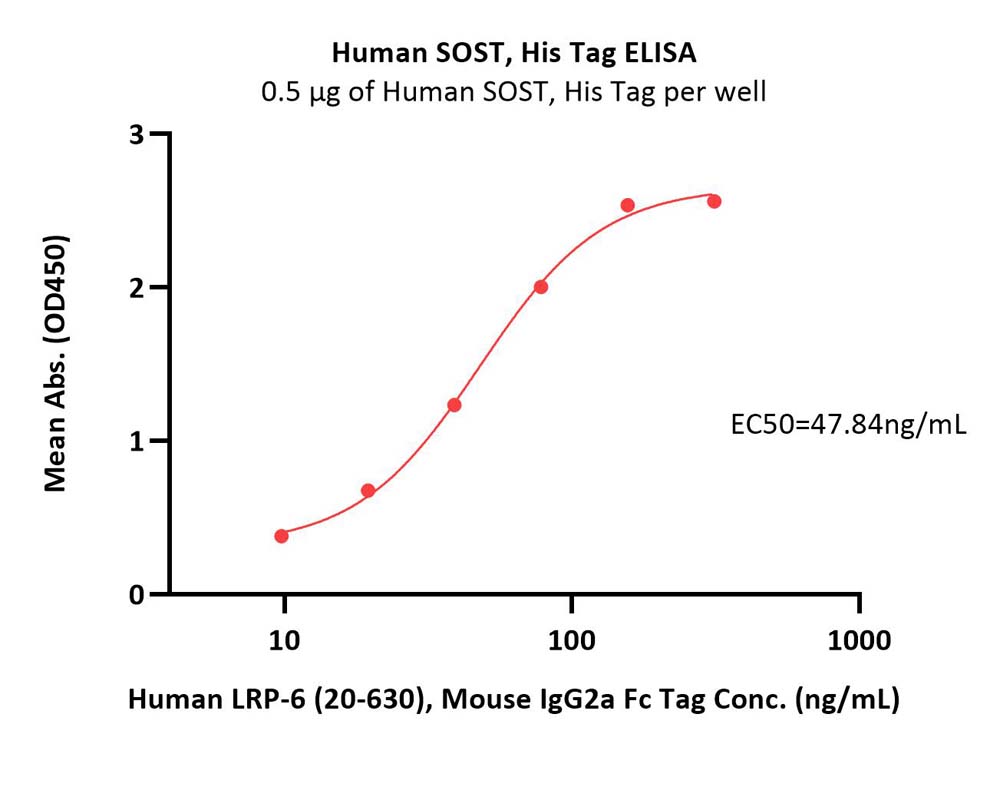
|

Immobilized Human SOST, His Tag (Cat. No. HST-H5245) at 5 μg/mL (100 μL/well) can bind Human LRP-6 (20-630), Mouse IgG2a Fc Tag (Cat. No. LR6-H5253) with a linear range of 10-78 ng/mL (QC tested).
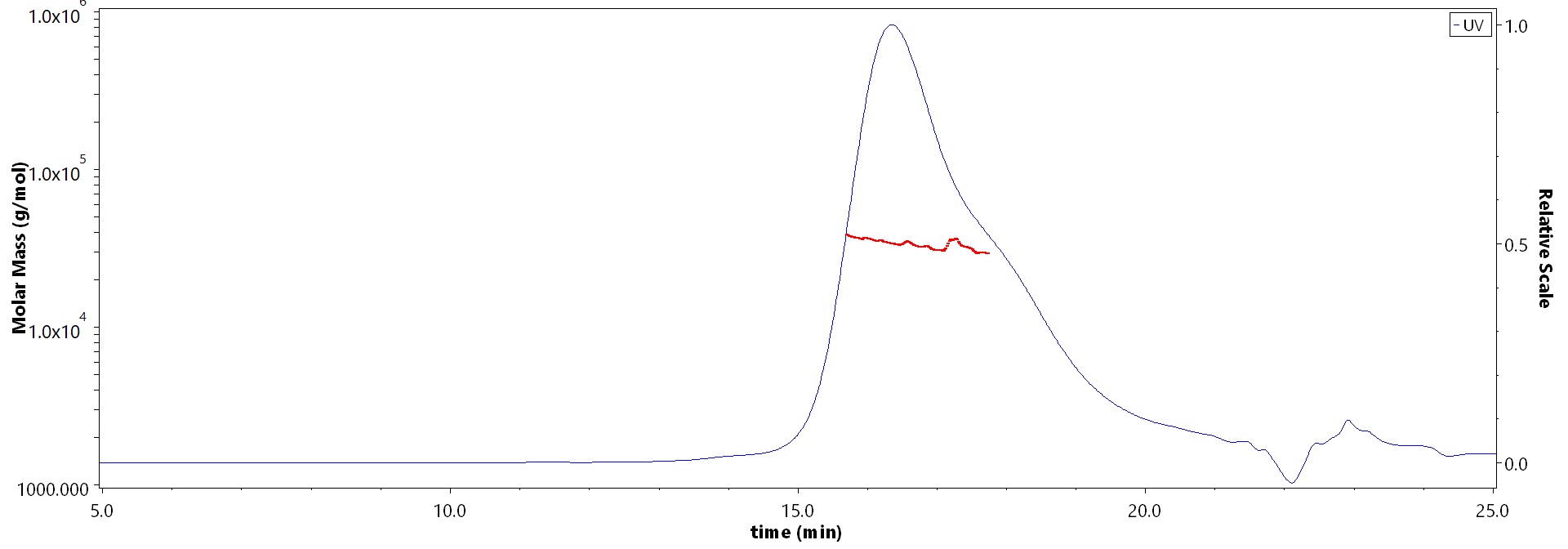
The purity of Human SOST, His Tag (Cat. No. HST-H5245) is more than 85% and the molecular weight of this protein is around 25-40 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Romosozumab | CDP-7851; AMG-785 | Approved | Evenity, EVENITY | Japan | Osteoporosis | Amgen Inc | 2019-01-08 | Osteoporosis; Bone Diseases, Metabolic; Osteoporosis, Postmenopausal; Osteogenesis Imperfecta; Fractures, Bone | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Setrusumab | UX143; NOV-3; MOR-05813; BPS-804 | Phase 3 Clinical | Morphosys Ag, Novartis Pharma Ag | Bone Diseases, Metabolic; Osteoporosis; Hypophosphatasia; Osteogenesis Imperfecta; Renal Insufficiency, Chronic | Details |
| AGA-2118 | AGA-2118 | Phase 2 Clinical | Angitia Biomedicines Ltd | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| Resugosbart | SHR-1222 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd, Suzhou Suncadia Biopharmaceuticals Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Osteoporosis; Osteoporosis, Postmenopausal | Details |
| AGA-2115 | AGA-2115; AGA2115 | Phase 1 Clinical | Angitia Biopharmaceuticals (Guangzhou) Ltd | Osteogenesis Imperfecta | Details |
| Blosozumab | LY-2541546; TST002; TST-002 | Phase 1 Clinical | Eli Lilly And Company | Osteoporosis; Osteoporosis, Postmenopausal | Details |
This web search service is supported by Google Inc.







