
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| TTR-H5223 | Human | Human Transthyretin / Prealbumin Protein, His Tag |  |
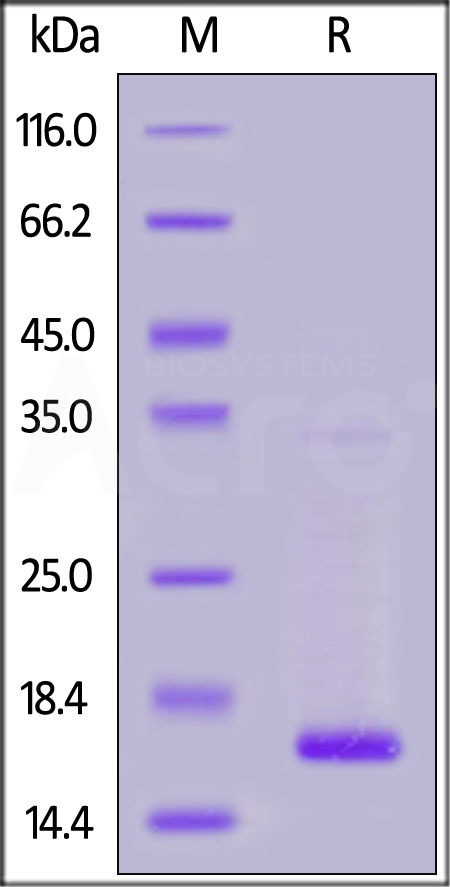
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Patisiran | SAR-438037; GENZ-438027; ALN-TTR02; ALN-18328 | Approved | Alnylam Pharmaceuticals Inc | Onpattro | United States | Amyloid Neuropathies, Familial | Alnylam Pharmaceuticals Inc | 2018-08-10 | Amyloid Neuropathies, Familial; Amyloidosis, Familial; Polyneuropathies; Cardiomyopathies; Amyloidosis; Transthyretin amyloidosis; Amyloid Neuropathies | Details |
| Vutrisiran | ALN-TTRSC02 | Approved | Alnylam Pharmaceuticals Inc | AMVUTTRA | United States | Amyloid Neuropathies, Familial; Transthyretin amyloidosis | Alnylam Pharmaceuticals Inc | 2022-06-13 | Amyloid Neuropathies, Familial; Amyloidosis, Familial; Cardiomyopathies; Amyloidosis; Transthyretin amyloidosis | Details |
| Acoramidis | AG-10; ALXN-2060; ALXN 2060; BBP-265 | Approved | Stanford University | Attruby | United States | Transthyretin amyloidosis | Bridgebio Pharma Inc | 2024-11-22 | Heart Diseases; Polyneuropathies; Cardiomyopathies; Amyloidosis; Transthyretin amyloidosis; Amyloid Neuropathies | Details |
| Inotersen sodium | ISIS-420915; GSK-2998728 | Approved | Ionis Pharmaceuticals Inc | Tegsedi | EU | Amyloidosis | Akcea Therapeutics Ireland Ltd | 2018-07-05 | Adenomatous Polyposis Coli; Amyloid Neuropathies, Familial; Amyloidosis; Transthyretin amyloidosis | Details |
| Tafamidis | PF-6291826; Fx-1006A; PF-06291826; FX-1006A | Approved | Pfizer Inc | 维万心, VYNDAMAX | United States | Heart Diseases | Foldrx Pharmaceuticals Inc | 2019-05-03 | Heart Diseases; Transthyretin amyloidosis | Details |
| Eplontersen | IONIS-TTR-LRx; AKCEA-TTR-LRx; ION-682884; ION-TTR-LRx | Approved | Ionis Pharmaceuticals Inc | WAINUA | United States | Transthyretin amyloidosis | Astrazeneca Ab | 2023-12-21 | Amyloid Neuropathies, Familial; Cardiomyopathies; Transthyretin amyloidosis | Details |
| Tafamidis Meglumine | FX-1006A; PF-6291826; Fx-1006A; PF-06291826 | Approved | Pfizer Inc | 维达全, Vyndaqel | EU | Amyloidosis | Pfizer Europe Ma Eeig | 2011-11-16 | Amyloid Neuropathies, Familial; Immunoglobulin Light-chain Amyloidosis; Cardiomyopathies; Amyloidosis; Amyloid Neuropathies; Transthyretin amyloidosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| ALXN-2220 | ALXN2220; ALXN-2220; NI006; NI-006 | Phase 3 Clinical | AstraZeneca global R & D (China) Co Ltd | Cardiomyopathies; Transthyretin amyloidosis | Details |
| Nexiguran ziclumeran | NTLA-2001 | Phase 3 Clinical | Intellia Therapeutics Inc | Amyloidosis, Familial; Amyloid Neuropathies, Familial; Peripheral Nervous System Diseases; Metabolism, Inborn Errors; Polyneuropathies; Genetic Diseases, Inborn; Cardiomyopathies; Metabolic Diseases; Amyloidosis; Nervous System Diseases; Neuromuscular Diseases; Heredodegenerative Disorders, Nervous System; Transthyretin amyloidosis; Neurodegenerative Diseases; Amyloid Neuropathies | Details |
| YOLT-201 | YOLT-201 | Phase 2 Clinical | Yoltech Therapeutics Co Ltd | Polyneuropathies; Genetic Diseases, Inborn; Cardiomyopathies; Transthyretin amyloidosis | Details |
| NNC6019-0001 | NNC6019-0001; NNC-6019; PRX-004; NN6019 | Phase 2 Clinical | Novo Nordisk A/S | Cardiomyopathies; Transthyretin amyloidosis | Details |
| NI-006 | NI-006 | Phase 1 Clinical | Neurimmune Ag | Cardiomyopathies; Transthyretin amyloidosis | Details |
| Nucresiran | ALNTTRsc-04 | Phase 1 Clinical | Alnylam Pharmaceuticals Inc | Transthyretin amyloidosis | Details |
This web search service is supported by Google Inc.







