
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| VE1-H5253 | Human | Human VEGF R1 Protein, Fc Tag |  |
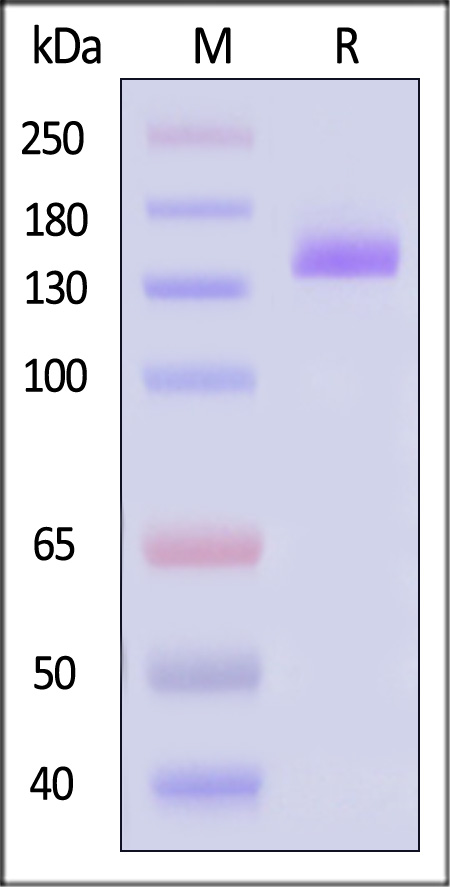
|
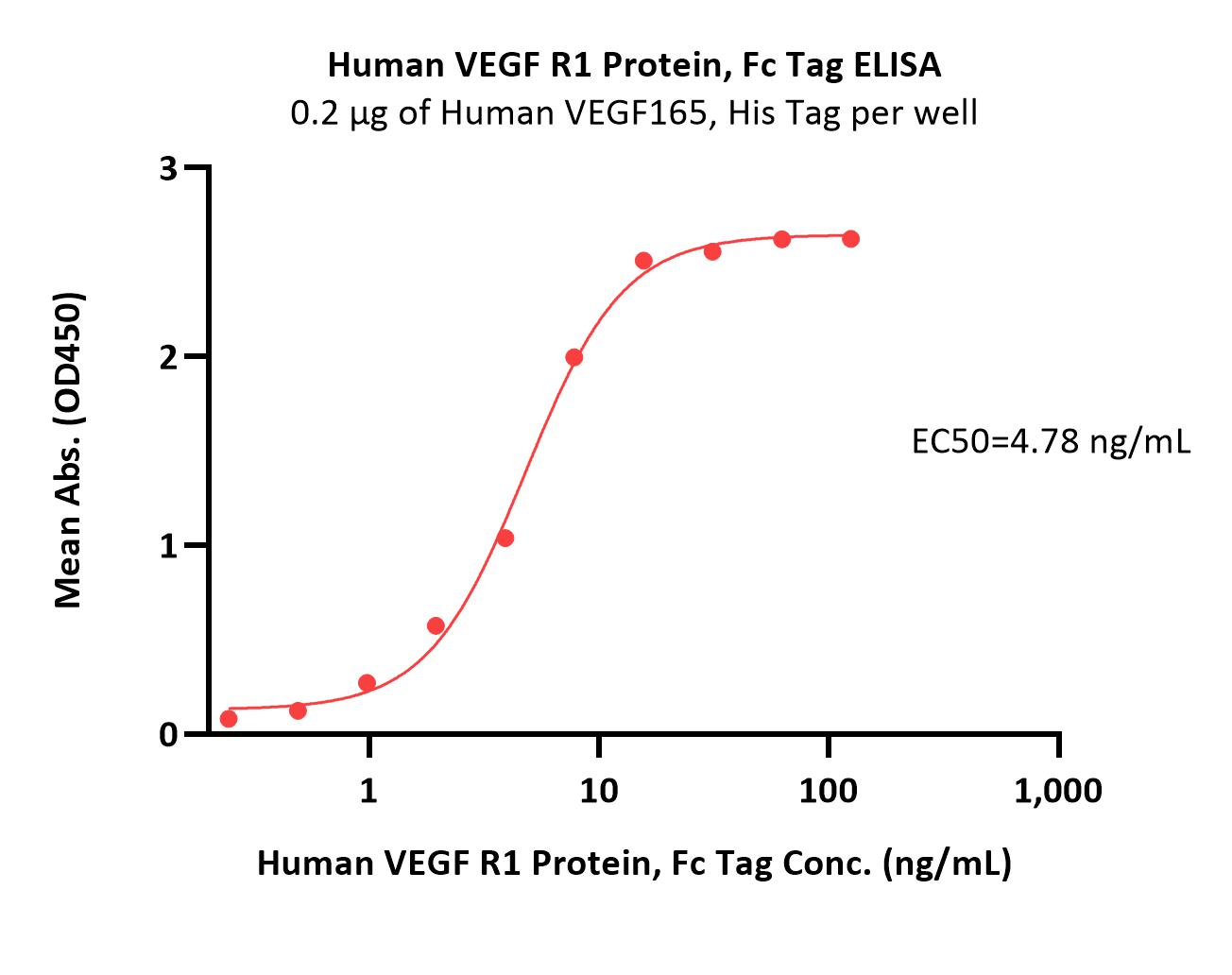
|
| VE1-H52H9 | Human | Human VEGF R1 / Flt-1 Protein, His Tag (MALS verified) |  |

|
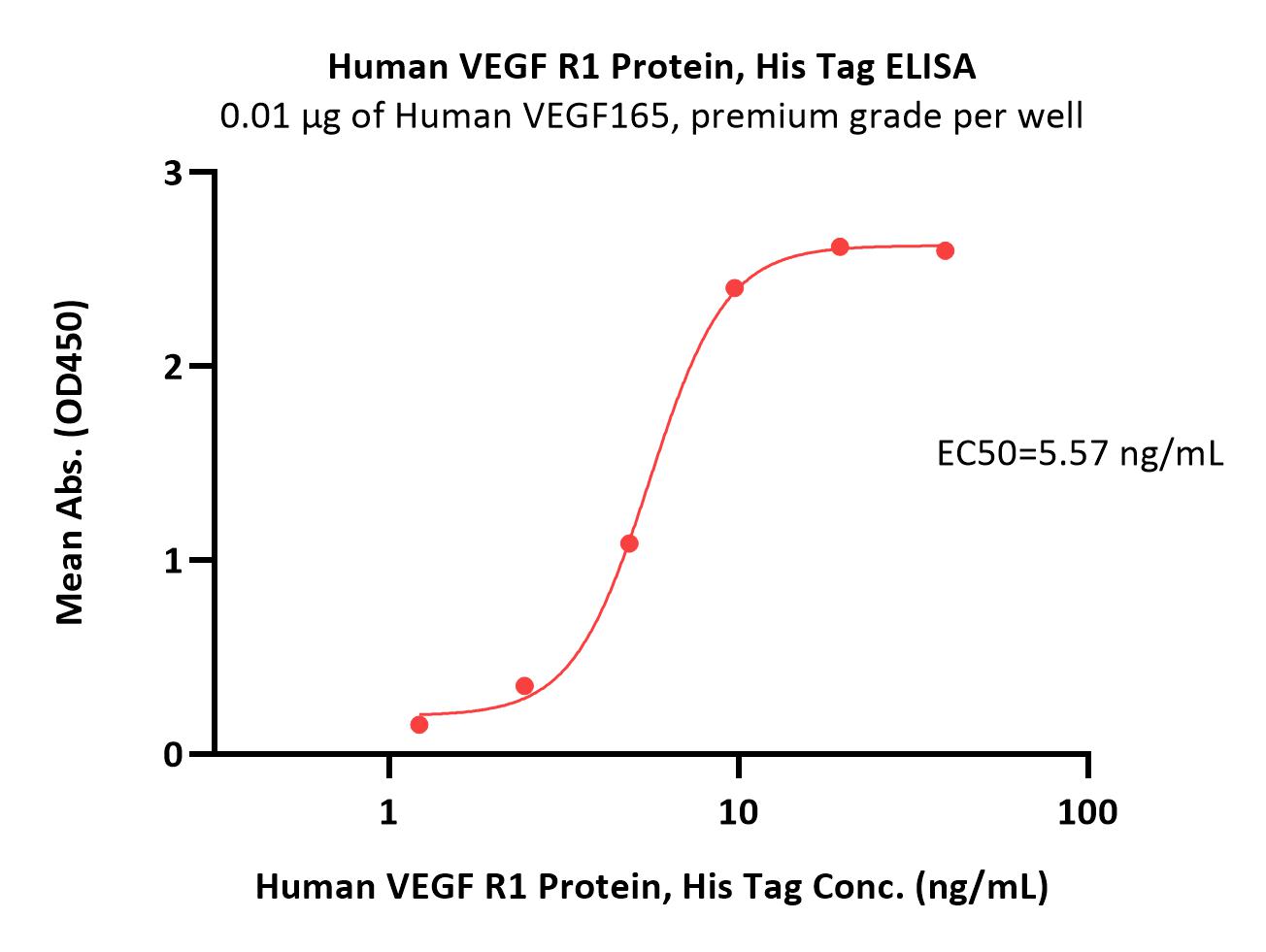
|
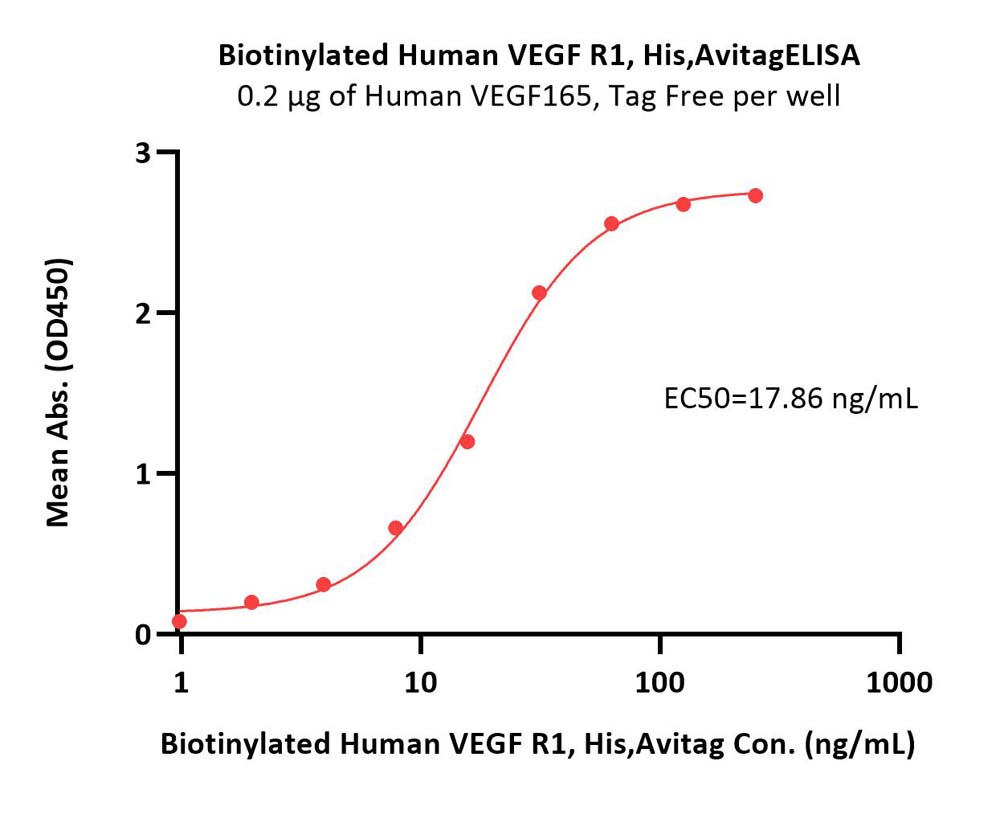
| VE1-H82E3 | Human | Biotinylated Human VEGF R1 / Flt-1 Protein, His,Avitag™ |  |
|
|
| VE1-M5256 | Mouse | Mouse VEGF R1 / Flt-1 Protein, Mouse IgG2a Fc Tag, low endotoxin |  |
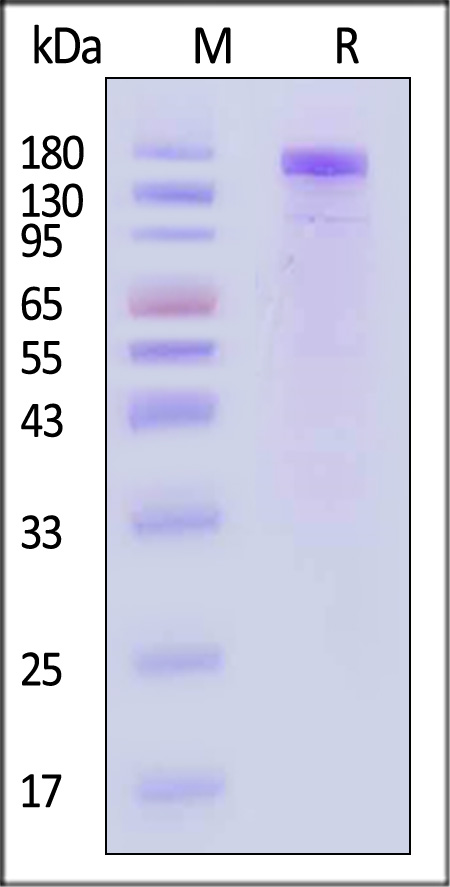
|
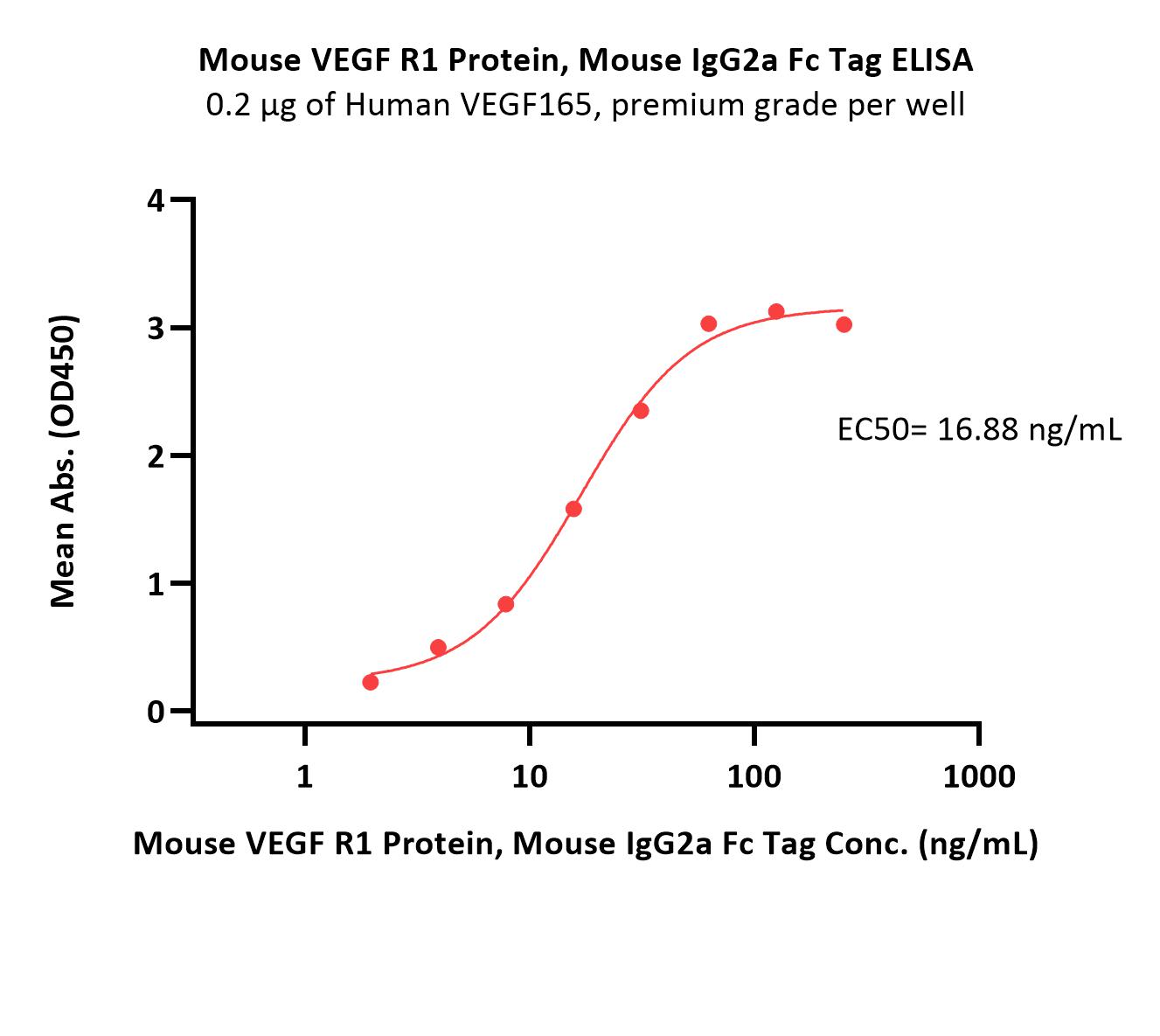
|

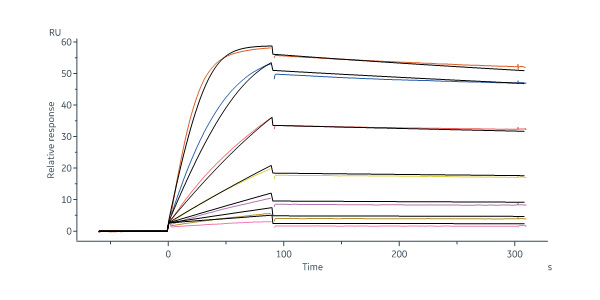
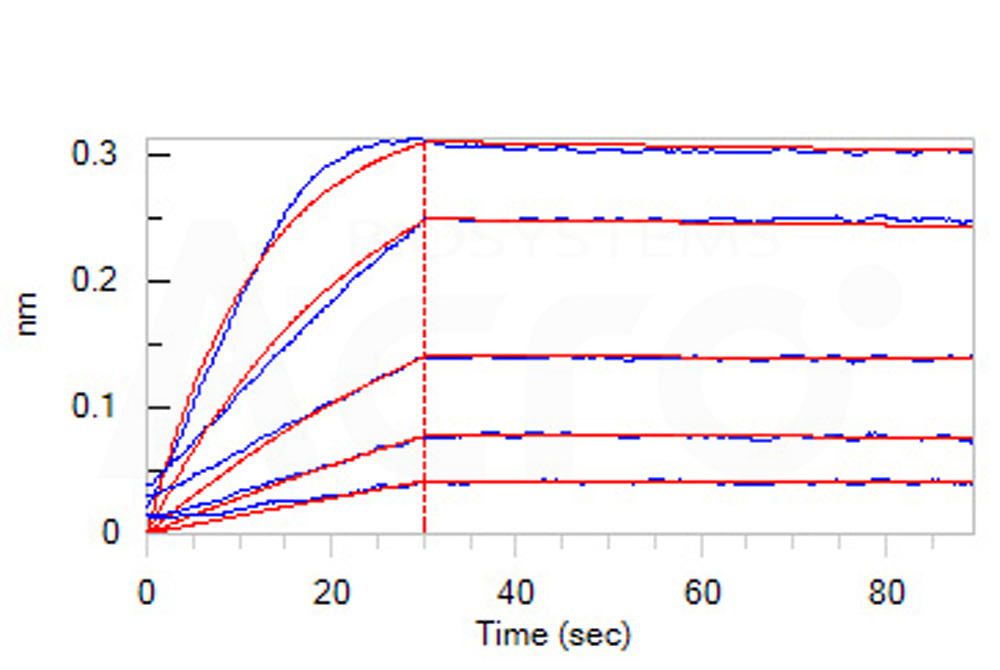
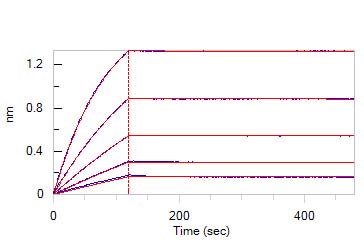
Human VEGF R1 Protein, Fc Tag (Cat. No. VE1-H5253) captured on Protein A Chip can bind Human VEGF-B, His Tag (Cat. No. VE6-H5225) with an affinity constant of 0.52 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Thyroid Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Urologic Neoplasms; Gastrointestinal Stromal Tumors; Hepatic Insufficiency; Bone Neoplasms; Endometrial Neoplasms; Gallbladder Neoplasms; Fallopian Tube Neoplasms; Medullary thyroid cancer (MTC); Sarcoma, Alveolar Soft Part; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Melanoma; Osteoma; Gastroenteropancreatic neuroendocrine tumor; Small Cell Lung Carcinoma; Solid tumours; Ovarian Neoplasms; Head and Neck Neoplasms; Leiomyosarcoma; Esophageal Neoplasms; Stomach Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Biliary Tract Neoplasms; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Neuroendocrine Tumors; Sarcoma, Synovial; Liver Diseases; Sarcoma; Bile Duct Diseases; Nasopharyngeal Carcinoma | Details |
| Lenvatinib Mesylate | MK-7902; ER-203492-00; E-7080 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | United States | Thyroid Neoplasms | Eisai Inc | 2015-02-13 | Neuroendocrine Tumors; Paraganglioma; Thyroid Cancer, Papillary; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Cholangiocarcinoma; Osteosarcoma; Solid tumours; Carcinoma, Adenoid Cystic; Adenocarcinoma, Follicular; Liver Diseases; Thyroid Carcinoma, Anaplastic; Adenocarcinoma of Lung; Neoplasms; Renal Insufficiency; Pheochromocytoma; Esophageal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib | PF-01367866; AG-13736; AG-013736 | Approved | Pfizer Inc | 英立达, Inlyta | United States | Carcinoma, Renal Cell | Pf Prism Cv | 2012-01-27 | Lung Neoplasms; Carcinoma, Ductal; Uveal melanoma; Breast Neoplasms; Sarcoma; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Hepatic Insufficiency; Thyroid Neoplasms; Prostatic Neoplasms, Castration-Resistant; Leukemia, Myeloid, Acute; Lymphoma; Carcinoma, Pancreatic Ductal; Adenocarcinoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Melanoma; Paraganglioma; Nasopharyngeal Neoplasms; Ovarian Neoplasms; Solid tumours; Carcinoma, Renal Cell; Carcinoid Tumor; Pheochromocytoma; Carcinoma; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Kidney Neoplasms; Adrenal Cortex Neoplasms; Skin Neoplasms; Myelodysplastic Syndromes; Glioblastoma; Pancreatic Neoplasms; Mesothelioma; Neuroendocrine Tumors; Carcinoma, Adenoid Cystic | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | United States | Colorectal Neoplasms | Bayer Healthcare Pharmaceuticals Inc | 2012-09-27 | Leukemia, Myeloid, Acute; Sarcoma, Ewing; Colorectal Neoplasms; Gastrointestinal Stromal Tumors; Bone Neoplasms; Peritoneal Neoplasms; Bile Duct Neoplasms; Esophageal adenocarcinoma; Lung Neoplasms; Thyroid Neoplasms; Fallopian Tube Neoplasms; Osteosarcoma; Thymoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Neoplasm Metastasis; Adenocarcinoma; Somatostatinoma; Gastrointestinal Neoplasms; Melanoma; Meningioma; Neoplasms; Solid tumours; Liver Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Insulinoma; Carcinoma, Islet Cell; Hemangiosarcoma; Rectal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Colonic Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Adenoma; Glucagonoma; Carcinoma, Adenoid Cystic; Gastrinoma; Sarcoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | United States | Idiopathic Pulmonary Fibrosis | Boehringer Ingelheim Gmbh | 2014-10-15 | Astrocytoma; Sarcoma; Prostatic Neoplasms; Breast Neoplasms; systemic sclerosis-associated interstitial lung disease; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Colorectal Neoplasms; Silicosis; Genital Neoplasms, Female; Hepatic Insufficiency; Oligodendroglioma; Gliosarcoma; Uterine Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Squamous Cell; Leukemia, Myeloid, Acute; Appendiceal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Endometrioid; Carcinoma, Hepatocellular; Glioblastoma; Solid tumours; Telangiectasia, Hereditary Hemorrhagic; Carcinoma, Renal Cell; Endometrial Stromal Tumors; Carcinoid Tumor; Radiation Pneumonitis; Esophageal Neoplasms; Rejection of lung transplantation; Idiopathic Pulmonary Fibrosis; Neoplasms; Ovarian Neoplasms; Small Cell Lung Carcinoma; Colonic Neoplasms; Scleroderma, Systemic; Lung Diseases, Interstitial; Pulmonary Fibrosis; Mesothelioma; Multiple Myeloma; Asbestosis; Neuroendocrine Tumors | Details |
| Tivozanib | Kil-8951; AV-951; KRN-951; ASP-4130; KHK-4951; KHK4951; UNII-172030934T | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda, FOTIVDA | EU | Carcinoma, Renal Cell | Recordati Netherlands BV | 2017-08-24 | Wet Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Fallopian Tube Neoplasms; Metastatic breast cancer; Hepatic Insufficiency; Peritoneal Neoplasms; Colorectal Neoplasms; Bile Duct Neoplasms; Biliary Tract Neoplasms; Sarcoma; Cholangiocarcinoma; Breast Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Solid tumours | Details |
| Selpercatinib | ARRY-192; Ret-IN-1; LOXO-292; LY-3527723 | Approved | Array Biopharma | Retevmo, Retsevmo | United States | Medullary thyroid cancer (MTC); Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms | Loxo Oncology Inc | 2020-05-08 | Medullary thyroid cancer (MTC); Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Thyroid Cancer, Papillary; Carcinoma, Non-Small-Cell Lung; Glioma; Lung Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Squamous Cell; Thyroid Neoplasms; Hepatic Insufficiency; Neuroblastoma; Histiocytosis, Langerhans-Cell; Sarcoma, Ewing; Osteosarcoma; Fibrosarcoma; Sarcoma; Myofibromatosis; Wilms Tumor; Neoplasms; Thyroid Carcinoma, Anaplastic; Colonic Neoplasms; Hepatoblastoma; Rhabdoid Tumor; Renal Insufficiency; Medulloblastoma; Ependymoma; Rhabdomyosarcoma; Hematologic Neoplasms; Solid tumours | Details |
| Sunitinib Malate | PNU-290940AD; PHA-290940AD; SCAI-003; SU-010398; SU-11248; GB-102; PHA-290940; PNU-290940; SU-011248-L-malate salt | Approved | Pfizer Inc | Sutent, 索坦 | United States | Carcinoma, Renal Cell; Gastrointestinal Stromal Tumors | Cppi Cv | 2006-01-26 | Kidney Neoplasms; Skin Melanoma; Teratoma; Liver Neoplasms; Ovarian Neoplasms; Ependymoma; Head and Neck Neoplasms; Lymphoma, T-Cell, Peripheral; Solid tumours; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Leiomyosarcoma; Lymphoma, B-Cell; HIV Infections; Fibrosarcoma; Intestinal Neoplasms; Fibromatosis, Aggressive; Leukemia; Lymphoma, B-Cell, Marginal Zone; Carcinoma, Renal Cell; Pheochromocytoma; Stomach Neoplasms; Abdominal Neoplasms; Pelvic Neoplasms; Polycythemia Vera; Thoracic Neoplasms; Esophageal Neoplasms; Carcinoma, Islet Cell; Carcinoma; Histiocytoma, Malignant Fibrous; Hemangioblastoma; Squamous Cell Carcinoma of Head and Neck; Leukemia, Hairy Cell; Nose Neoplasms; Hypopharyngeal Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic neuroendocrine tumors (pNET); Plasmacytoma; Neoplasms; Pancreatic Neoplasms; Glioblastoma; Lymphoma, Large B-Cell, Diffuse; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Myelodysplastic Syndromes; Carcinoma, Transitional Cell | Details |
| Plitidepsin | APLD | Approved | Pharma Mar Sa | Aplidin | Australia | Multiple Myeloma | null | 2018-12-11 | Solid tumours; Leukemia; Coronavirus Disease 2019 (COVID-19); Liposarcoma; Multiple Myeloma; Post-Acute COVID-19 Syndrome; Prostatic Neoplasms; Primary Myelofibrosis; Lymphoma; Angioimmunoblastic T-cell Lymphoma | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | United States | Carcinoma, Renal Cell; Sarcoma | Novartis Pharma Ag | 2009-10-19 | Osteosarcoma; Germinoma; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Colorectal Neoplasms; Uterine Cervical Diseases; Genital Neoplasms, Female; Gliosarcoma; Gastrointestinal Stromal Tumors; Peritoneal Neoplasms; Chondrosarcoma, Extraskeletal Myxoid; Lung Neoplasms; Nasopharyngeal Carcinoma; Medullary thyroid cancer (MTC); Brain Neoplasms; Urethral Neoplasms; Psoriasis; Gastrinoma; Neuroblastoma; Prostatic Neoplasms; Breast Neoplasms; Neoplasms, Germ Cell and Embryonal; von Hippel-Lindau Disease; Thyroid Cancer, Papillary; Neoplasm Metastasis; Breast Neoplasms, Male; Uterine Cervical Neoplasms; Endodermal Sinus Tumor; Paraganglioma; Melanoma; Macular Degeneration; Carcinoma, Hepatocellular; Sarcoma; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Choriocarcinoma; Uterine Neoplasms; Lymphoma; Carcinoma, Neuroendocrine; Glioma; Carcinoma, Embryonal; Skin Melanoma; Neoplasms; Herpes Genitalis; Hemangiosarcoma; Squamous Cell Carcinoma of Head and Neck; Insulinoma; Pheochromo | Details |
| Cabozantinib S-malate | XL-184; BMS-907351; RO7047650; RO-7047650 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Thyroid Neoplasms; Carcinoma, Neuroendocrine | Exelixis Inc | 2012-11-29 | Neuroblastoma; Thyroid Neoplasms; Hepatic Insufficiency; Astrocytoma; Peritoneal Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Gliosarcoma; Urethral Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Cholangiocarcinoma; Neurofibroma, Plexiform; Brain Neoplasms; Adenocarcinoma, Clear Cell; Carcinoma, Adenosquamous; Medullary thyroid cancer (MTC); Sarcoma, Ewing; Sarcoma; Osteosarcoma; Sarcoma, Alveolar Soft Part; Meningioma; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Paraganglioma; Neoplasms, Germ Cell and Embryonal; Carcinoma, Hepatocellular; Prostatic Neoplasms; Glioma; Brain metastases; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Squamous Cell; Lymphoma; Carcinoma, Neuroendocrine; Fallopian Tube Neoplasms; Carcinoma, Renal Cell; Adenoma, Islet Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Renal Insufficiency; Hepatoblastoma; Pheochromocytoma; Carcinoid Tumor; Carcinoma, Merkel Cell; Rejection of liver transp | Details |
| Fruquintinib | HMPL-013; TAK-113 | Approved | Hutchison Medipharma Ltd | FRUZAQLA, Aiyoute, 爱优特, Elunate | Mainland China | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Pancreatic Neoplasms; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Metastatic breast cancer; Endometrial Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Breast Neoplasms; Sarcoma; Neoplasms; Solid tumours; Colonic Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Renal Insufficiency; Biliary Tract Neoplasms | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | United States | Carcinoma, Renal Cell | Bayer Healthcare Pharmaceuticals Inc | 2005-12-01 | Liver Neoplasms; Leukemia, Myelogenous, Chronic; Leiomyosarcoma; Rhabdomyosarcoma; Solid tumours; Ovarian Neoplasms; Fibromatosis, Aggressive; Kidney Neoplasms; Leukemia, Myeloid; Head and Neck Neoplasms; Skin Melanoma; Lymphoma, B-Cell, Marginal Zone; Lymphoma, T-Cell, Peripheral; Leukemia, Erythroblastic, Acute; Squamous Cell Carcinoma of Head and Neck; Carcinoma; Histiocytoma, Malignant Fibrous; Carcinoma, Renal Cell; Stomach Neoplasms; Rectal Neoplasms; Carcinoma, Islet Cell; Insulinoma; Vipoma; Hemangiosarcoma; Esophageal Neoplasms; Wilms Tumor; Hypertension, Portal; Thyroid Carcinoma, Anaplastic; Neoplasms; Myelodysplastic Syndromes; Leukemia, Myelomonocytic, Chronic; Pancreatic Neoplasms; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Lymphomatoid Granulomatosis; Carcinoma, Transitional Cell; Colonic Neoplasms; Salivary Gland Neoplasms; Lymphoma, Large B-Cell, Diffuse; Multiple Endocrine Neoplasia Type 2a; Lymphoma, Large-Cell, Immunoblastic; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Solid tumours; Ovarian Neoplasms; Leiomyosarcoma; Leukemia; Liver Neoplasms; Optic Nerve Glioma; Skin Melanoma; Head and Neck Neoplasms; Biliary Tract Neoplasms; Medulloblastoma; Lymphoma, B-Cell, Marginal Zone; Ependymoma; Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Carcinoma, Renal Cell; Carcinoma; Spinal Cord Neoplasms; Stomach Neoplasms; Rectal Neoplasms; Leukemia, Hairy Cell; Rhabdoid Tumor; Squamous Cell Carcinoma of Head and Neck; Cystadenoma, Serous; Cystadenocarcinoma, Serous; Cystadenocarcinoma, Mucinous; Glioblastoma; Carcinoma, Transitional Cell; Neoplasms; Pancreatic Neoplasms; Carcinoma, Ovarian Epithelial; Leukemia-Lymphoma, Adult T-Cell; Hormone-Resistant Prostate Neoplasms; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Colonic Neoplasms; Carcinoma, Verrucous; Nasopharyngeal Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hypopharyngeal Neoplasms; Hodgkin Disease; Myelodysplastic Syndromes; Lymphoma, Large-Cell, Immunoblastic; Salivary Gland Neoplasms; Lymphomatoid Granulomatosi | Details |
| Sitravatinib | MGCD-516; IND-155305; MGCD-0516; MG-91516; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Endometrial Neoplasms; Lung Neoplasms; Metastatic breast cancer; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Carcinoma, Squamous Cell; Hepatic Insufficiency; Ureteral Neoplasms; Solid tumours; Lung Diseases; Liposarcoma; Uveal melanoma; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Kidney Neoplasms; Biliary Tract Neoplasms | Details |
| Axitinib intravitreal implant (Ocular Therapeutix) | OTX-TKI | Phase 3 Clinical | Ocular Therapeutix Inc | Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| AUR-109 | ODM-203; AUR-109 | Phase 2 Clinical | Orion Corp | Ovarian Neoplasms; Liver Neoplasms; Kidney Neoplasms; Solid tumours; Carcinoma, Renal Cell; Urinary Bladder Neoplasms; Pulmonary Fibrosis; Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms | Details |
| Lucitanib | S-80881; AL-3810; CO-3810; E-3810; S-80881-2 | Phase 2 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Nasopharyngeal Carcinoma; Genital Neoplasms, Female; Colorectal Neoplasms; Thymus Neoplasms; Lung Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| OCV-101 | OCV-101; OTS-11101 | Phase 2 Clinical | Oncotherapy Science, Inc | Pancreatic Neoplasms | Details |
| Axitinib Implant(Aerie) | AR-14034 SR; AR-14034 | Phase 2 Clinical | Aerie Pharmaceuticals Inc | Macular Degeneration | Details |
| Sorafenib Tosylate/Comekibart | MG-D-1609 | Phase 2 Clinical | Metagone Biotech Inc | Solid tumours | Details |
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| KHK-4951 | Phase 2 Clinical | Kyowa Kirin | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| Axitinib injectable suspension (Clearside Biomedical) | CLS-1002; CLS-011-A; CLS011A; CLS-AX | Phase 2 Clinical | Clearside Biomedical Inc | Macular Degeneration | Details |
| nintedanib (Avalyn Pharma) | Phase 1 Clinical | Avalyn Pharma Inc | Idiopathic Pulmonary Fibrosis; Pulmonary Fibrosis | Details | |
| Amorphous Formulation of Sorafenib in Mesoporous Magnesium Carbonate | DPH001; DPH-001 | Phase 1 Clinical | Disruptive Pharma AB | Details | |
| MNKD-201 | MNKD-201; MKND-201 | Phase 1 Clinical | MannKind Corp | Idiopathic Pulmonary Fibrosis | Details |
| Inhaled Nintedanib – dry powder (Avalyn Pharma) | AP02; AP 02 DP; AP-02; AP02-DP | Phase 1 Clinical | Avalyn Pharma Inc | Idiopathic Pulmonary Fibrosis; Respiratory Tract Diseases; Lung Diseases, Interstitial; Pulmonary Fibrosis | Details |
| CBP-4888 | CBP-4888 | Phase 1 Clinical | Pre-Eclampsia | Details | |
| Dual-targeting VEGFR1 and PD-L1 CAR-T cells Therapy (Sichuan University) | Phase 1 Clinical | Sichuan University | Serositis; Ascites | Details | |
| Sorafenib tosylate (Bio-Synectics) | BS-104 | Clinical | Bio-Synectics Inc | Details |
This web search service is supported by Google Inc.







