
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| CD2-AHFM8 | Mouse | APC-Labeled Monoclonal Anti-Human CD2 Antibody, Mouse IgG1 (RPA-2.10) (0.03% Proclin) |

|
||
| CD2-PCFM8 | Mouse | PE-Labeled Monoclonal Anti-Human CD2 Antibody, Mouse IgG1 (RPA-2.10) (0.03% Proclin) |

|
||
| HGS-S256 | Rabbit | Recombinant Monoclonal Anti-CD2 Antibody, Rabbit (5E2) | |||
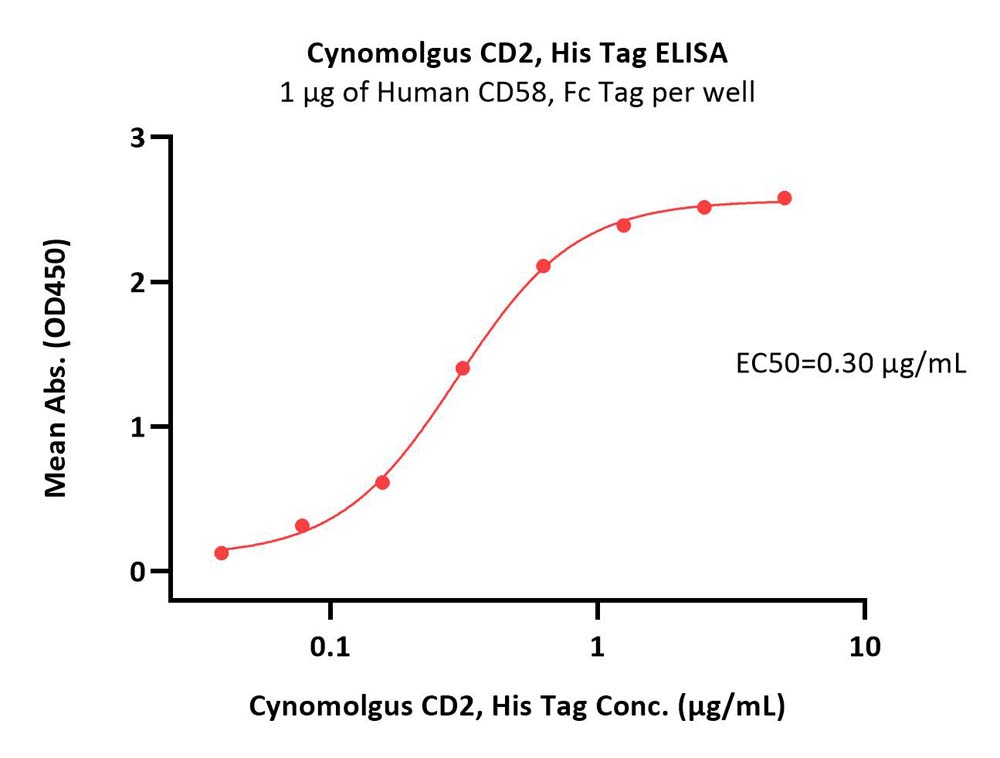
| CD2-C52H5 | Cynomolgus | Cynomolgus CD2 / SRBC Protein, His Tag |  |

|
|
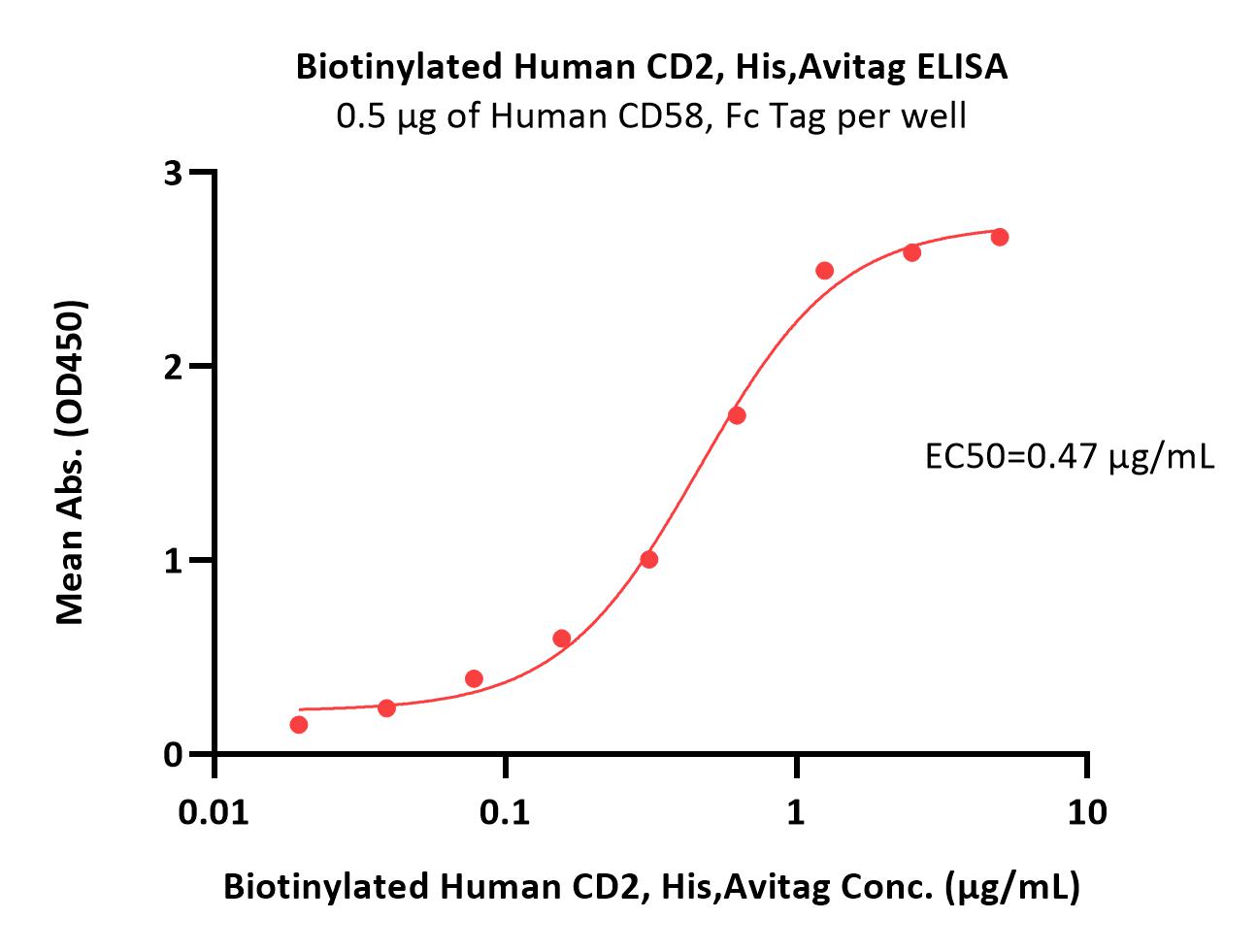
| CD2-H82E6 | Human | Biotinylated Human CD2 / SRBC Protein, His,Avitag™ (MALS verified) |  |

|
|
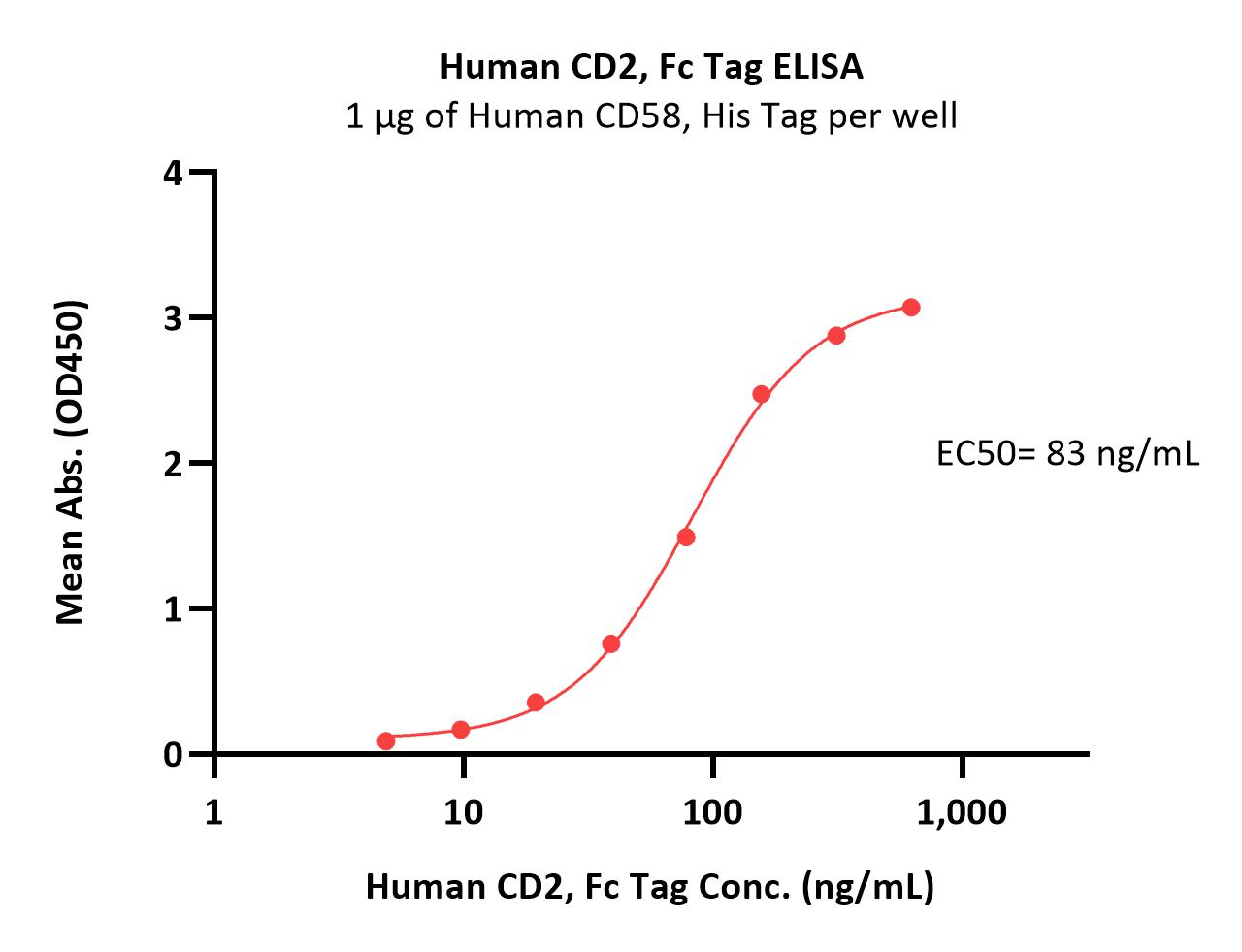
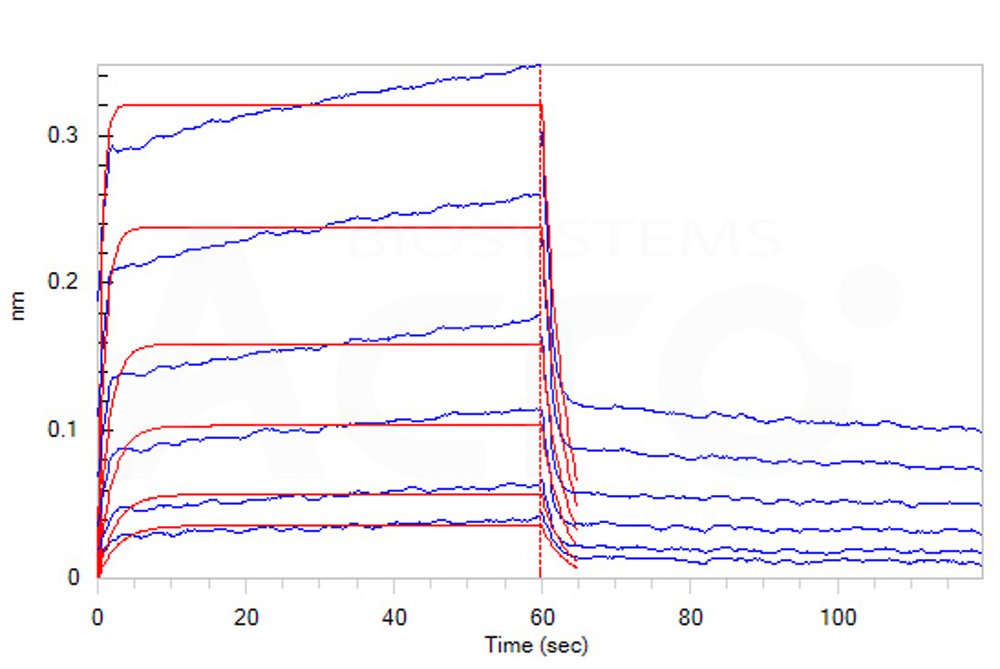
| CD2-H5258 | Human | Human CD2 / SRBC Protein, Fc Tag |  |

|
|
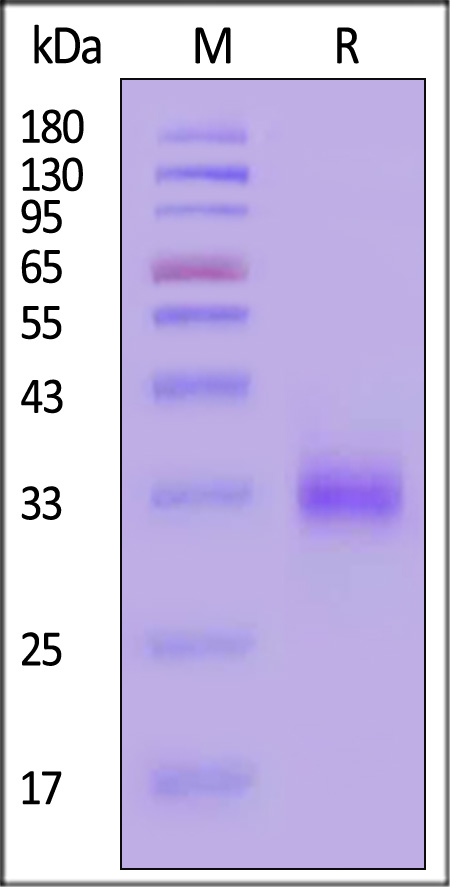
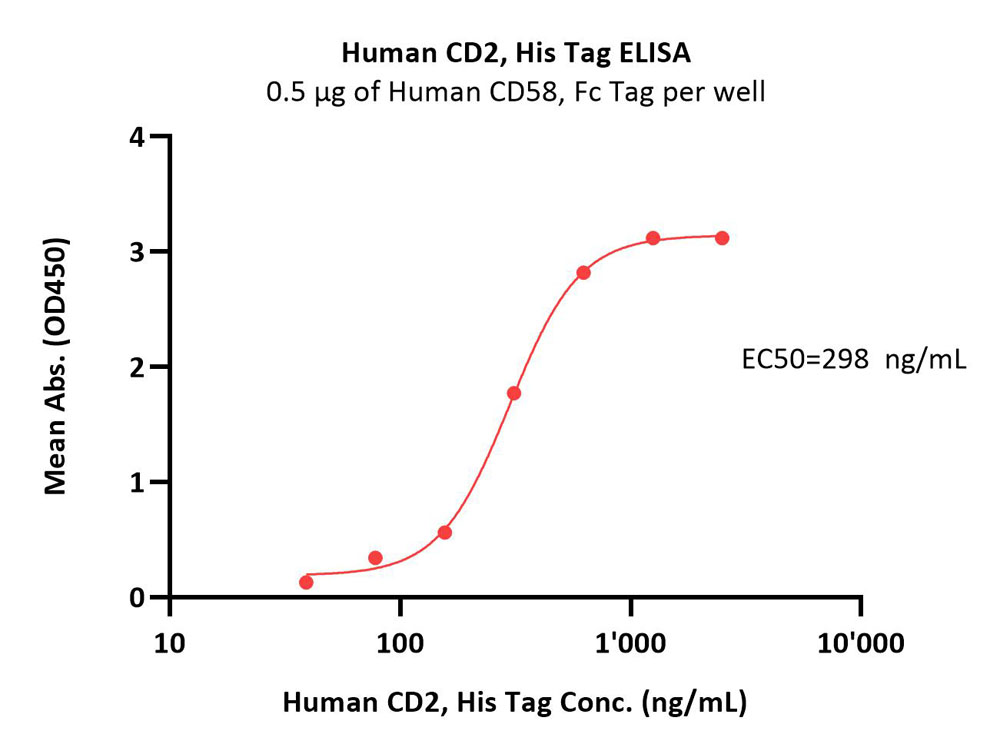
| CD2-H5226 | Human | Human CD2 / SRBC Protein, His Tag (MALS verified) |  |
|
|

Flow cytometric analysis of Human peripheral blood lymphocytes staining with APC-Labeled Monoclonal Anti-Human CD2 Antibody, Mouse IgG1 (RPA-2.10) (Cat. No. CD2-AHFM8) at 1:50 dilution (2 μL of the antibody stock solution corresponds to labeling of 1e6 PBMCs in a final volume of 100 µL), compared with APC Mouse IgG1, κ Isotype Ctrl Antibody. APC signal was used to evaluate the binding activity (QC tested).

Flow cytometric analysis of Human peripheral blood lymphocytes staining with PE-Labeled Monoclonal Anti-Human CD2 Antibody, Mouse IgG1 (RPA-2.10) (Cat. No. CD2-PCFM8) at 1:50 dilution (2 μL of the antibody stock solution corresponds to labeling of 1e6 PBMCs in a final volume of 100 µL), compared with PE Mouse IgG1, κ Isotype Ctrl Antibody. PE signal was used to evaluate the binding activity (QC tested).

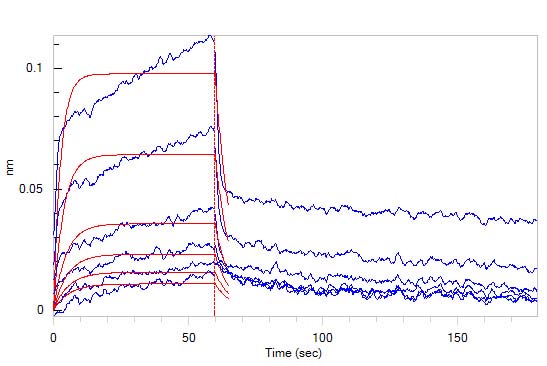
Loaded Human CD2, Fc Tag (Cat. No. CD2-H5258) on Protein A Biosensor, can bind Human CD58, His Tag (Cat. No. LF3-H5225) with an affinity constant of 2.3 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Siplizumab | MEDI-507; TCD-601 | Phase 2 Clinical | Catholic University Of Louvain, Itb-Med Llc | Multiple Myeloma; Amyotrophic Lateral Sclerosis; Kidney Failure, Chronic; Plaque psoriasis; Anemia, Sickle Cell; Lymphoma; Lymphoma, T-Cell; Hidradenitis Suppurativa; Hepatitis, Autoimmune; Lymphoma, Extranodal NK-T-Cell; Psoriasis; Cholangitis, Sclerosing; Diabetes Mellitus, Type 1; Liver Diseases; Myelodysplastic Syndromes; Neoplasms; Graft vs Host Disease; Kidney Diseases; Liver Cirrhosis; Renal Insufficiency; Rejection of liver transplantation; Leukemia; End Stage Liver Disease; Rejection of renal transplantation | Details |
| SBT-11-5301 | SBT115301 | Phase 1 Clinical | Sonoma Biotherapeutics Inc | Autoimmune Diseases | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Neoplasms; Lupus Erythematosus, Systemic; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
This web search service is supported by Google Inc.







