
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































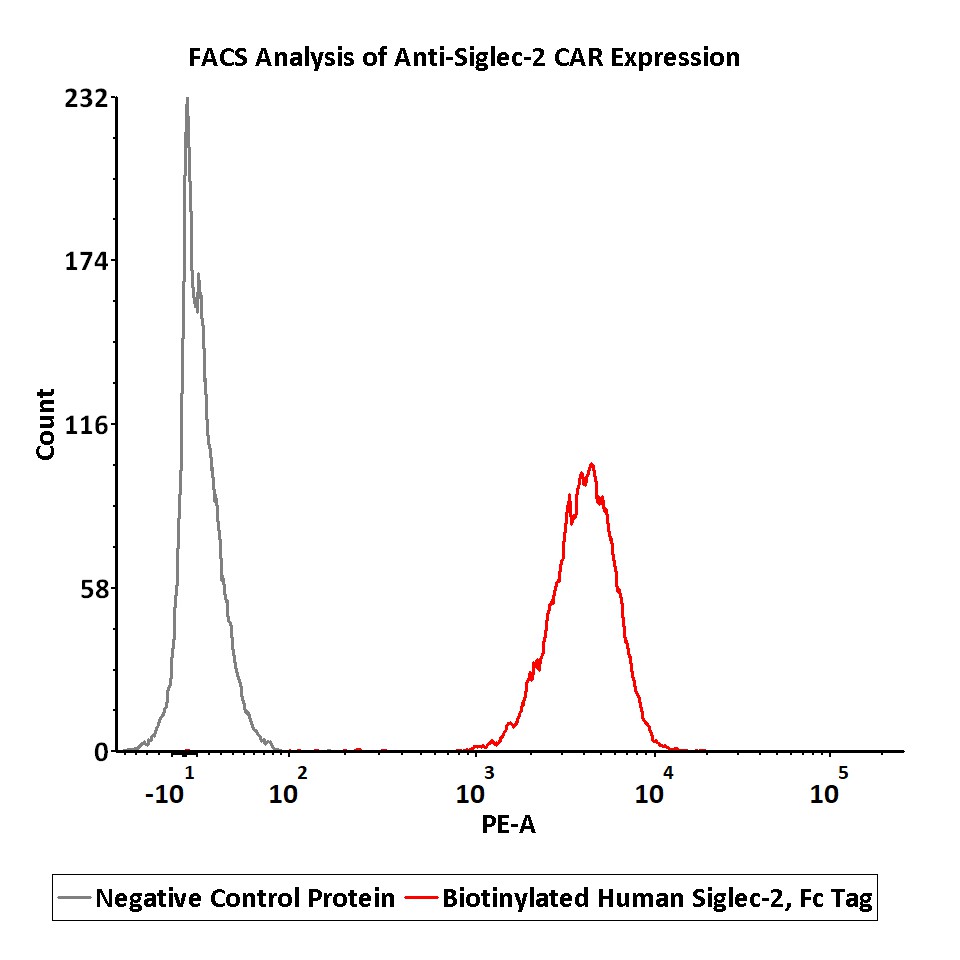
2e5 of anti-Siglec-2 CAR-293 cells were stained with 100 μL of 0.3 μg/mL of Biotinylated Human Siglec-2 Protein, Fc Tag, premium grade, primary amine labeling (Cat. No. CD2-H5254) and negative control protein respectively, washed and then followed by PE-SA and analyzed with FACS (QC tested).
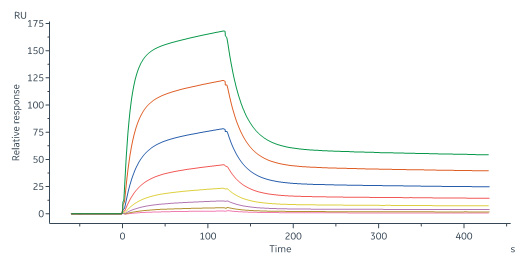
Monoclonal Anti-Human Siglec-2 Antibody, Human IgG1 captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human Siglec-2 (176-687), His Tag (Cat. No. SI2-H52H8) with an affinity constant of 0.121 μM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Moxetumomab pasudotox | HA-22; GCR-8015; CAT-8015 | Approved | Astrazeneca Plc | Lumoxiti | United States | Leukemia, Hairy Cell | Innate Pharma | 2018-09-13 | Leukemia; Leukemia, Hairy Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Prolymphocytic; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Inotuzumab Ozogamicin | WAY-207294; PF-5208773; CMC-544; PF-05208773 | Approved | Wyeth Pharmaceutical Co Ltd, Celltech Group plc | Besponsa | EU | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Pfizer Europe Ma Eeig | 2017-06-28 | Lymphoma, B-Cell; Leukemia, Myelogenous, Chronic; Hematologic Neoplasms; Leukemia; Neoplasm, Residual; Hematopoietic stem cell transplantation (HSCT); Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Leukemia, B-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| AUTO-3 | AUTO-3 | Phase 2 Clinical | University College London | Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti CD22 CAR-T cell therapy (Shanghai Yake Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd, The First Affiliated Hospital Of Zhejiang University School Of Medicine, Beijing Gao Boren Hospital Co Ltd, Chengdu USino Technology Biology Co Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CART22-cells | CART22-cells; JJO686 | Phase 2 Clinical | Novartis Pharma Ag, University Of Pennsylvania | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| Anti-CD22-CAR T cell therapy (Kecellitics Biotech) | Phase 2 Clinical | Kecellitics Biotech Company Ltd | Lymphoma, B-Cell; Leukemia; Lymphoma | Details | |
| Epratuzumab-cys-tesirine | ADCT-602 | Phase 2 Clinical | Adc Therapeutics Sa | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19/22-CAR vector-transduced T cell therapy (Chinese PLA General Hospital) | Phase 2 Clinical | People'S Liberation Army General Hospital Military Service | Leukemia; Lymphoma | Details | |
| Anti-CD22 CAR-T cell therapy (No. 307 Hospital) | Phase 2 Clinical | The Fifth Medical Center Of Pla General Hospital (Formerly 307 Hospital) | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| UCART-20x22 | UCART-20x22 | Phase 2 Clinical | Cellectis Sa | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| TAC-001 | TAAC-001 | Phase 2 Clinical | Tallac Therapeutics Inc | Solid tumours | Details |
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-T | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma | Details |
| KQ-2002 | KQ-2002 | Phase 2 Clinical | Novatim Immune Therapeutics (Zhejiang) Co Ltd | Lymphoma, B-Cell; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| Fourth-gen CAR T Cells Targeting CD19/CD22 therapy(Essen Biotech) | Phase 2 Clinical | Essen Biotech | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoma; Central Nervous System Lymphoma | Details | |
| CD19/CD22 Bispecific CAR-T Cell Therapy(Beijing Tongren Hospital) | Phase 2 Clinical | Beijing Tongren Hospital, Cmu | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Firicabtagene autoleucel | CRG-022 | Phase 2 Clinical | Cargo Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Lymphoma, Follicular; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19/CD22 CAR-T cell therapy (The First Affiliated Hospital Of Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Dual Anti-CD22/CD19 Chimeric Antigen Receptor-directed T Cells (CART2219.1) therapy (KK Women's and Children's Hospital) | Phase 2 Clinical | Kk Women'S And Children'S Hospital | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| anti-CD22-CAR-transduced T cells(Southwest Hospital) | Phase 2 Clinical | Southwest Hospital | Leukemia; Lymphoma | Details | |
| CD19/22 Bi-specific CAR-T Cell Therapy(Shenzhen Geno-Immune Medical Institute) | 4SCAR19/22 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| Anti-CD19 and anti-CD22 CAR-T cell therapy(IASO Biotherapeutics) | CT-120 | Phase 2 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| CD19/CD22 dual-target CAR-T (Yake biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19/CD22 dual-target CAR-T cell therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| HY-004 (Juventas) | HY-004 (Juventas) | Phase 2 Clinical | Juventas Cell Therapy Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| SCRI-CAR22v2 | Phase 2 Clinical | Seattle Children'S Hospital | Leukemia; Lymphoma | Details | |
| CD19/CD22 CAR-T cell therapy (Federal Research Institute of Pediatric Hematology, Oncology and Immunology) | Phase 2 Clinical | Federal Research Institute of Pediatric Hematology Oncology and Immunology | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Burkitt Lymphoma | Details | |
| UCART-22 | UCART-22 | Phase 2 Clinical | Cellectis Sa | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Autologous CD19/CD22 chimeric antigen receptor T-cell therapy (MD Anderson Cancer Center) | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Neoplasm, Residual; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| TRPH-222 | CD22-4AP; TRPH-222; CAT-02-106 | Phase 1 Clinical | Catalent Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma; Lymphoma, Non-Hodgkin | Details |
| Anti-CD19/CD22 CAR-T cell therapy (Jiao Tong University) | Phase 1 Clinical | Shanghai Jiaotong University, Shanghai General Hospital | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Dual anti-CD19/CD22 CAR‐T cell therapy (B-cell malignancies, Stanford University) | Phase 1 Clinical | Stanford University, Orca Biosystems Inc | Neoplasm, Residual; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19/CD22 CAR-T cell therapy (Shenzhen BinDeBio) | Phase 1 Clinical | Dehe Biotech Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19 and anti-CD22 CAR T cell therapy (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD22 CAR T cell therapy (Shanghai GeneChem) | Phase 1 Clinical | Shanghai Genechem Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19- and CD22 specific CAR (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Leukemia; Lymphoma | Details | |
| RD-102 | RD-102 | Phase 1 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse | Details |
| YT-19/22 | Phase 1 Clinical | China Immunotech Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| TriCAR T-cells | Phase 1 Clinical | Children'S Hospital Los Angeles | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| anti-CD19/anti-CD22 CAR T-cell therapy (MD Anderson Cancer Center/National Cancer Institute) | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Dual Anti-CD19/Anti-CD22 CAR-T Cell Therapy (Stanford University) | Phase 1 Clinical | Stanford University | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD10 CAR T-cell therapy (Zhujiang Hospital/Nanfang Hospital) | Phase 1 Clinical | Southern Medical University Zhujing Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD22/CD19 monoclonal antibody-toxin conjugate | Phase 1 Clinical | The University Of Texas Southwestern Medical Center | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| B-019 | B-019 | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| Anti-CD19 anti-CD22 bispecific chimeric antigen receptor T cell therapy (Hrain Biotechnology) | Phase 1 Clinical | Hrain Biotechnology Co Ltd | Leukemia, Lymphoid; Central Nervous System Lymphoma | Details | |
| CD19-CD22-Bispecific Chimeric Antigen Receptor (CAR) T Cell Therapy(St. Jude Children'S Research Hospital) | Phase 1 Clinical | St. Jude Children'S Research Hospital | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Autologous CD19/CD22 CAR T cells therapy(Instituto De Investigación Hospital Universitario La Paz) | Phase 1 Clinical | Instituto De Investigación Hospital Universitario La Paz | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| UB-VV400 | UB-VV400 | Phase 1 Clinical | Umoja BioPharma Inc, Iaso Biotherapeutics Ltd | Lymphoma, B-Cell; Autoimmune Diseases; Lymphoma, Non-Hodgkin | Details |
| BZE-2204 | BZE2204; BZE-2204 | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Lymphoma, B-Cell; Multiple Myeloma | Details |
| AutologousCD22 Chimeric Antigen Receptor (CAR)T Cells therapy(Stanford University) | Phase 1 Clinical | Stanford University, The Leukemia And Lymphoma Society | Leukemia; Lymphoma, B-Cell, Marginal Zone; Leukemia, Hairy Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Waldenstrom Macroglobulinemia; Burkitt Lymphoma | Details | |
| Donor-derived CD19 CAR Therapy Bridged Allo-HSCT and Sequential Donor-derived CD22 CAR Therapy(Beijing GoBroad Hospital) | Phase 1 Clinical | Beijing GoBroad Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Allogeneic CD22-directed CAR T Cell Therapy(Sana Biotechnology) | SC262; SC-262 | Phase 1 Clinical | Sana Biotechnology Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| AUTO-1-NG | AUTO-1-NG; AUTO1/22 | Phase 1 Clinical | Autolus Therapeutics Plc, University College London | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Burkitt Lymphoma | Details |
| Autologous CD22 targeting CAR-T cells therapy(British Columbia Cancer Agency) | CLIC-2201 | Phase 1 Clinical | British Columbia Cancer Agency | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| CD22 CAR-T Cell Therapy (Hebei Senlang Biotechnology) | Phase 1 Clinical | Hebei Senlang Biological Technology Co Ltd | Solid tumours; Sarcoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| REGN5837 | REGN5837; REGN-5837 | Phase 1 Clinical | Regeneron Pharmaceuticals Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| bispecific CD19/CD22 CAR T-cell therapy (University of Colorado) | Phase 1 Clinical | University Of Colorado | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| LCAR-AIO | LCAR-AIO; VHH CAR-T | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| ThisCAR-T-22(Fundamenta Therapeutics) | ThisCAR-T-22 | Phase 1 Clinical | Fundamenta Therapeutics Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| CD19x22 CAR T Cell Therapy (University Of Colorado Denver) | Phase 1 Clinical | University Of Colorado, Denver, Usa | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| CD19/CD20 Dual CAR-T Cell Therapy (China Immunotech) | HX-s001; YTS101; HX-s001/YTS101; HXYT-001 | Phase 1 Clinical | Beijing Qingyi Taike Pharmaceutical Technology Co Ltd, China Immunotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Anti-human CD19-CD22 T cell therapy | HR004 | Phase 1 Clinical | Hrain Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Central Nervous System Lymphoma | Details |
| Rezetamig | JNJ-8780; JNJ75348780; JNJ-75348780 | Phase 1 Clinical | Teneobio Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD22 CAR T cell therapy (Wuhan Bio-Raid) | Phase 1 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Lymphoma, B-Cell; Multiple Myeloma | Details | |
| CD20/CD22 dual Targeted CAR T-cell therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University, Shanghai YaKe Biotechnology Co Ltd | Hematologic Neoplasms | Details | |
| JCAR-018 | JCAR-018 | Phase 1 Clinical | National Cancer Institute, Opus Bio | Lymphoma, B-Cell; Lymphoma, Follicular; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic | Details |
| Anti-CD19 and anti-CD22 CAR T cell therapy (Hebei Senlang Biotechnology) | Phase 1 Clinical | Hebei Senlang Biological Technology Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| CTA-101 | CTA-101 | Phase 1 Clinical | Nanjing Bioheng Biotech Co Ltd, Nanjing Medical University, Xuzhou Medical University (Xzmu) | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| Anti-CD22 chimeric antigen receptor T cell therapy (Yake Biotechnology) | Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Anti CD19/CD22 chimeric antigen receptor T cell therapy (Yake Biotechnology) | Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
This web search service is supported by Google Inc.







