
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































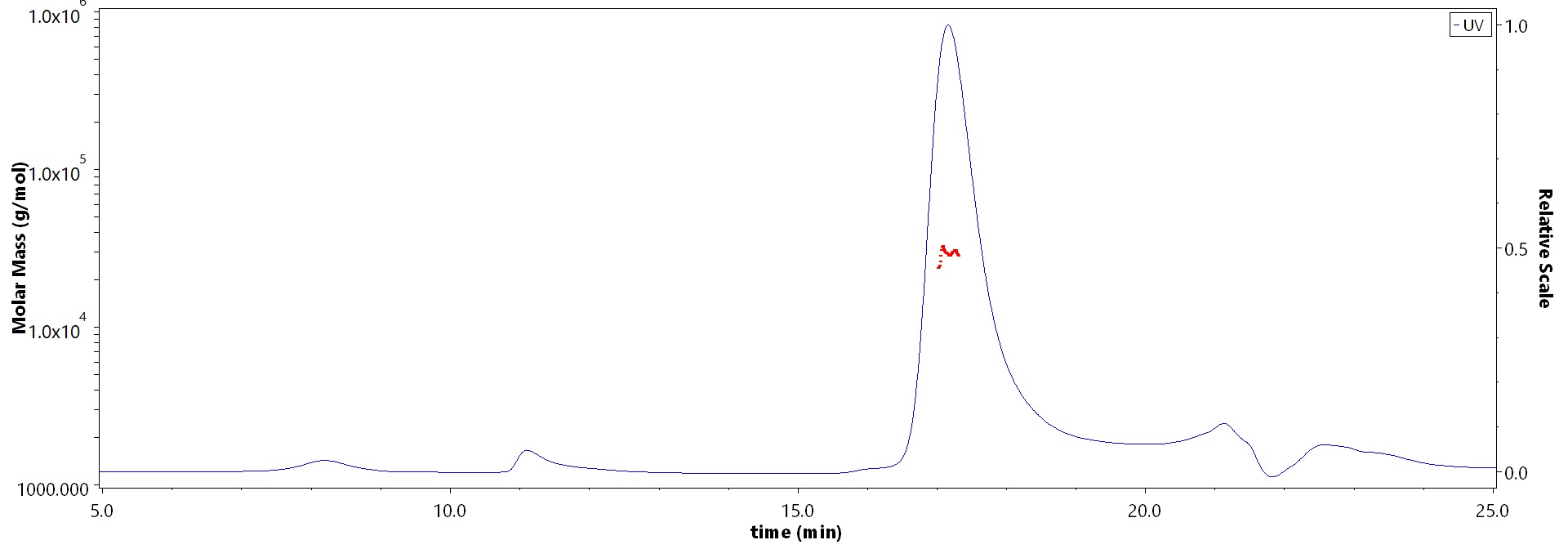
The purity of Human KRAS (2-185,G12D), His Tag (Cat. No. KRS-H51H4) is more than 90% and the molecular weight of this protein is around 24-34 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Pooled mutant-KRAS peptide vaccine (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins) | Phase 2 Clinical | Sidney Kimmel Comprehensive Cancer Center | Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| KRAS TCR-Transduced PBL(NIH) | Phase 2 Clinical | National Cancer Institute | Intestinal Neoplasms; Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Duodenal Neoplasms | Details | |
| JAB-23E73 | JAB-23E73 | Phase 2 Clinical | Jacobio Pharmaceuticals Group Co Ltd | Solid tumours; Pancreatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ZG-2001 | ZG2001; ZG-2001 | Phase 2 Clinical | Suzhou Zelgen Biopharmaceuticals Co Ltd | Solid tumours; Neoplasms | Details |
| ELI-002 | Amph-CpG-7909; ELI-002; ELI-002 2P; VED-002 | Phase 2 Clinical | Elicio Therapeutics | Solid tumours; Neoplasm, Residual; Ovarian Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Gallbladder Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| mDC3/8-KRAS Vaccine (University of Pennsylvania) | Phase 1 Clinical | University Of Pennsylvania | Carcinoma, Pancreatic Ductal | Details | |
| KrasG12D siRNA-loaded mesenchymal stromal cells-derived exosomes (Codiak BioSciences) | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Pancreatic Neoplasms; Carcinoma, Pancreatic Ductal | Details | |
| HB-700 | HB700; HB-700 | Phase 1 Clinical | Hookipa Pharma Inc | Pancreatic Neoplasms; Neoplasms; Colorectal Neoplasms; Lung Neoplasms | Details |
| PF-07985045 | PF-07985045; PF-5045 | Phase 1 Clinical | Pfizer Inc | Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| LY-4066434 | LY4066434; LY-4066434 | Phase 1 Clinical | Eli Lilly And Company | Solid tumours; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| BGB-53038 | BGB-53038 | Phase 1 Clinical | BeOne Medicines Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Esophageal adenocarcinoma; Lung Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms | Details |
| PF-07934040 | PF-4040; PF-07934040 | Phase 1 Clinical | Pfizer Inc | Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| BBO-10203 | BBO-10203 | Phase 1 Clinical | Bridgebio Pharma Inc | Solid tumours; Hyperglycemia; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Metastatic breast cancer | Details |
| ASP-4396 | ASP4396; ASP-4396 | Phase 1 Clinical | Astellas Pharma Inc | Solid tumours | Details |
| QTX-3544 | QTX-3544; QTX3544 | Phase 1 Clinical | Quanta Therapeutics Inc | Solid tumours; Neoplasms | Details |
| BI-3706674 | BI-3706674; BI3706674; BI 3706674 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms | Details |
| YL-17231 | YL-17231; YL17231; TEB-17231 | Phase 1 Clinical | YL-Pharma | Solid tumours; Neoplasms | Details |
| QTX-3034 | QTX3034; QTX-3034 | Phase 1 Clinical | Quanta Therapeutics Inc | Solid tumours; Neoplasms | Details |
| SY-5933 | SY-5933; SY5933 | Phase 1 Clinical | Shouyao Holding (Beijing) Co Ltd | Solid tumours; Neoplasms | Details |
| RMC-9805 | RM-036; RMC-9805 | Phase 1 Clinical | Revolution Medicines Inc | Solid tumours; Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| LUNA-18 | LUNA-18 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours | Details |
| BEBT-607 | C200825009-FP; BEBT-607 | Phase 1 Clinical | Guangzhou BeBetter Medicine Technology Co | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| BI-1701963 | BI-1701963 | Phase 1 Clinical | Forma Therapeutics Holdings Inc | Solid tumours; Colorectal Neoplasms | Details |
| Targeted KRAS TCR-T cell therapy(Immuxell Biotech) | Clinical | Shanghai Immuxell Biotech Ltd | Carcinoma, Pancreatic Ductal | Details | |
| CWG-59r | CWG-59r; CWG-59-r; CWG59r | Clinical | Cellworks Group | Neoplasms | Details |
| CWG-71b | CWG-71b; CWG-71-b; CWG71b | Clinical | Cellworks Group | Neoplasms | Details |
| CWG-89 | CWG-89 | Clinical | Cellworks Group | Neoplasms | Details |
| CWG-71a (Cellworks Group) | CWG-71a | Clinical | Cellworks Group Inc | Neoplasms | Details |
This web search service is supported by Google Inc.







