
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































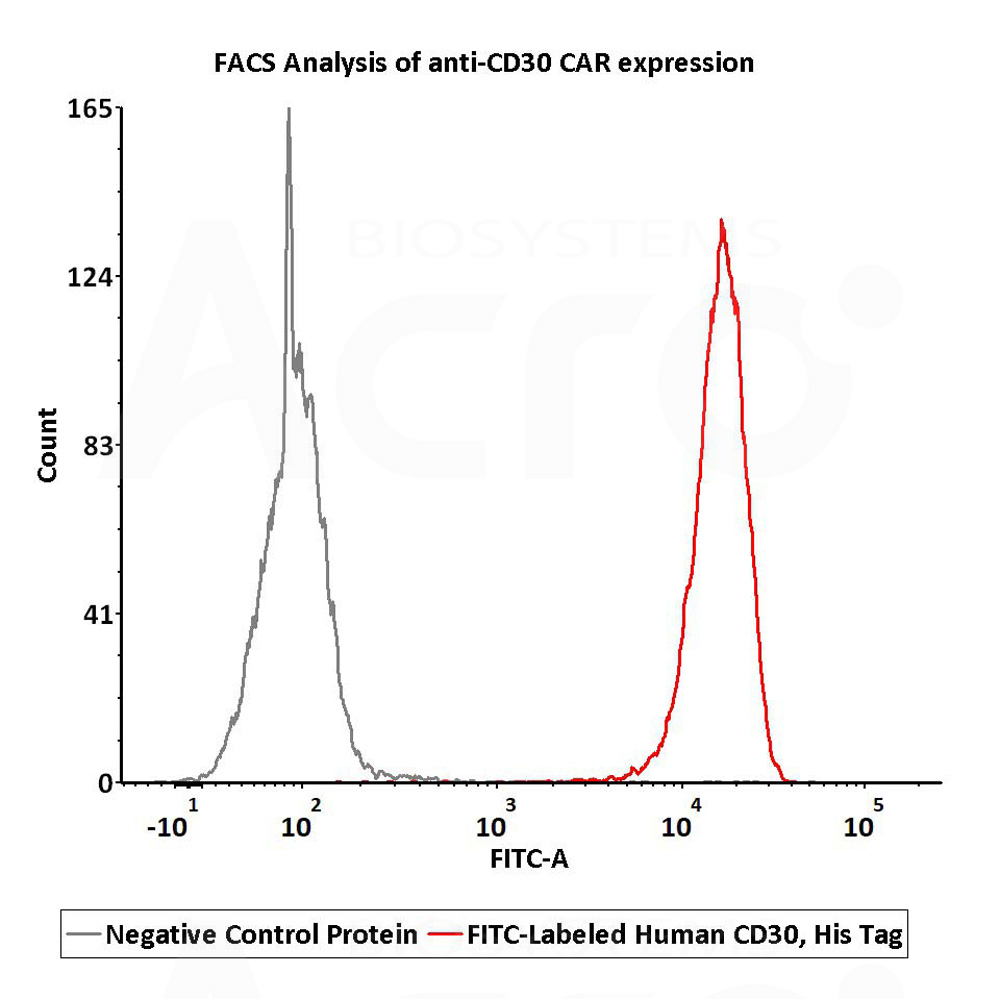
2e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1 μg/mL of FITC-Labeled Human CD30, His Tag (Cat. No.CD0-HF2H4) and negative control protein respectively, FITC signal was used to evaluate the binding activity (QC tested).
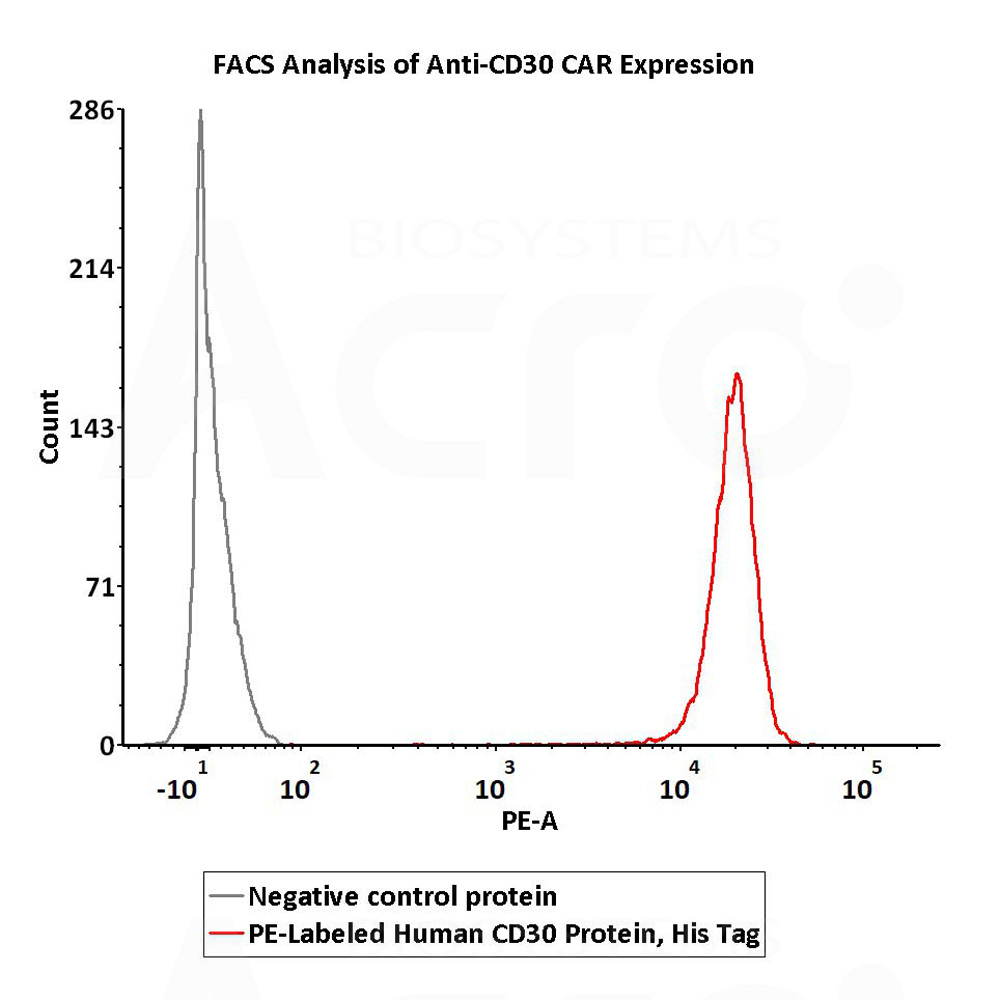
5e5 of anti-CD30 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD30, His Tag (Cat. No. CD0-HP2E3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
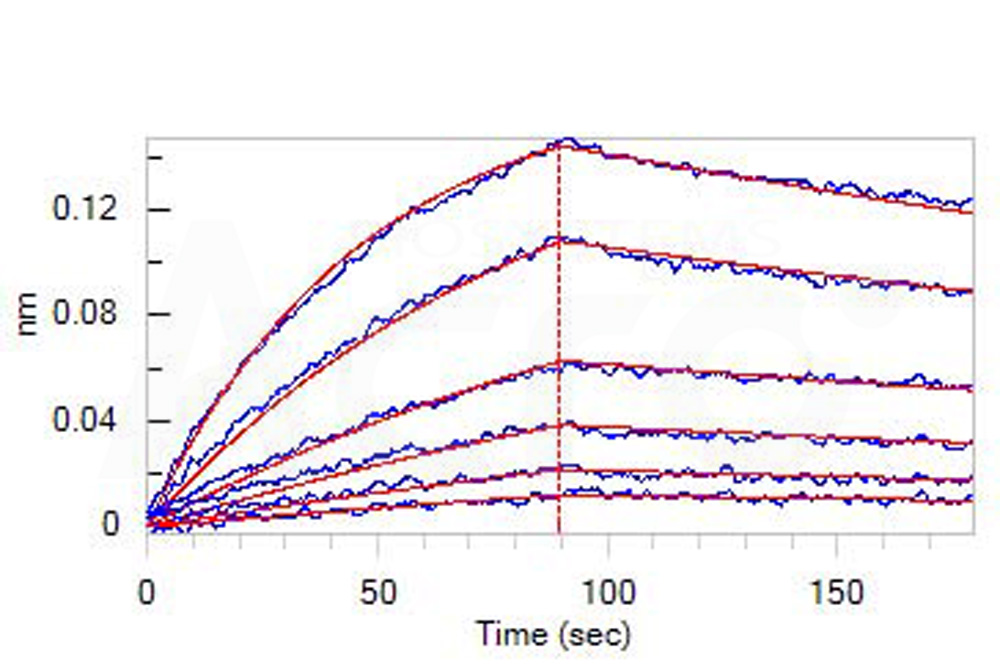
Loaded Human CD30 Ligand, His Tag (Cat. No. CDL-H524b) on HIS1K Biosensor, can bind Human CD30 Protein, Llama IgG2b Fc Tag (Cat. No. TN8-H5250) with an affinity constant of 55.5 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Brentuximab vedotin | SGN-30; SGN-35; cAC-10; SGD-1010; cAC10-vcMMAE; cAC10-Val-Cit-MMAE | Approved | Millennium Pharmaceuticals Inc, Seagen Inc | Adcetris, 安适利 | United States | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Seagen Inc | 2011-08-19 | Lymphoma, Large-Cell, Anaplastic; Lymphoma, Primary Cutaneous Anaplastic Large Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Sezary Syndrome; Lymphoma, T-Cell; Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Leukemia, Mast-Cell; Scleroderma, Diffuse; Lymphoma, Non-Hodgkin; Hidradenitis Suppurativa; Mycosis Fungoides; Lymphomatoid Papulosis; Sarcoma, Kaposi; Neoplasms, Germ Cell and Embryonal; Graft vs Host Disease; Lymphoma, T-Cell, Peripheral; Solid tumours; Hematologic Neoplasms; HIV Infections; Lymphoma, B-Cell; Anemia, Refractory, with Excess of Blasts; Carcinoma; Hodgkin Disease; Hematologic Diseases; Mastocytosis, Systemic; Scleroderma, Systemic; Lymphoma, Large B-Cell, Diffuse; Enteropathy-Associated T-Cell Lymphoma; Neoplasms; Myelodysplastic Syndromes; Mesothelioma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Acimtamig | AFM-13 | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin; Mycosis Fungoides | Details |
| ATLCAR.CD30 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 2 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, T-Cell, Peripheral; Lymphatic Diseases; Immunoproliferative Disorders; Neoplasms; Hodgkin Disease; Immune System Diseases; Lymphoproliferative Disorders; Lymphoma; Lymphoma, Non-Hodgkin; Neoplasms, Germ Cell and Embryonal | Details | |
| Autologous CD30+ CAR T Cells therapy(New York Medical College) | Phase 2 Clinical | New York Medical College, University Of North Carolina At Chapel Hill | Hodgkin Disease | Details | |
| Itezocabtagene autoleucel | TT-11; TT11 | Phase 2 Clinical | Tessa Therapeutics Ltd | Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic | Details |
| BRD-01 | BRD-01 | Phase 2 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms; Hodgkin Disease | Details |
| GEN3017 | GEN-3017 | Phase 2 Clinical | Genmab A/S | Hematologic Neoplasms; Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| CD30 biAb-AATC(The Medical College Of Wisconsin Nonprofit) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Leukemia; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic; Lymphoma; Lymphoma, T-Cell, Cutaneous | Details | |
| HSP-CAR-30 | HSP-CAR-30 | Phase 2 Clinical | Fundació Institut De Recerca De L | Hodgkin Disease; Lymphoma, T-Cell | Details |
| Anti-CD30 CAR T-cell therapy (General Hospital of the People's Liberation Army/Cellular Biomedicine) | CAR30-NKT; CBM-C30.1; CD30ScFv-CD8-CD137-CD3zeta | Phase 2 Clinical | Pla General Hospital | Hodgkin Disease | Details |
| Anti-CD30 chimeric antigen receptor T cell therapy (Immune cell) | Phase 1 Clinical | Immune Cell Inc | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| CD30.CAR-EBVST cell therapy (Baylor College of Medicine) | TT-11X | Phase 1 Clinical | Baylor College Of Medicine, Tessa Therapeutics Ltd | Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell | Details |
| EBV-specific-CAR.CD30 | EBV-specific-CAR.CD30; CAR.CD30 EBV-specific-CTLs | Phase 1 Clinical | Baylor College Of Medicine, Texas Children'S Hospital, Methodist Hospital System | Hodgkin Disease; Lymphoma, Non-Hodgkin | Details |
| Anti-CD30 CAR T-cell therapy (Wuhan Bio-Raid) | Phase 1 Clinical | Wuhan BioRaid Biotechnology Co Ltd | Lymphoma, T-Cell, Peripheral; Hematologic Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Hodgkin Disease; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Angioimmunoblastic T-cell Lymphoma | Details | |
| Recombinant chimeric anti-CD30 monoclonal antibody-MCC-DM1 | F0002-ADC; B-006(SCP) | Phase 1 Clinical | Shanghai Crosslink Pharmaceutical R & D Co Ltd, Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Hematologic Neoplasms; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details |
| CD30.CAR | H-27721; CD30.CAR | Phase 1 Clinical | Baylor College Of Medicine | Lymphoma, B-Cell; Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic; Lymphoma, Non-Hodgkin | Details |
| Anti-CD20/CD30-CAR-T Cell therapy(Shanghai Tongji Hospital) | Phase 1 Clinical | Shanghai Tongji Hospital | Lymphoma, B-Cell; Leukemia, B-Cell; Lymphoma | Details | |
| CD30-Targeted LCAR-HL30 Cells therapy(Legend Biotechnology) | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| SGN-35C | SGN-35C | Phase 1 Clinical | Seagen Inc | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Lymphoma; Lymphoma, Large-Cell, Anaplastic | Details |
| SGN-35T | SGN-35T; SGN-CD30C; PF-08046045 | Phase 1 Clinical | Seagen Inc | Solid tumours; Lymphoma, T-Cell, Peripheral; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic; Lymphoma, T-Cell, Cutaneous | Details |
| Anti-CD30 CAR T-cell therapy | Phase 1 Clinical | Minghui (Nanjing) Gene Biotechnology Co Ltd | Hodgkin Disease; Lymphoma, Large-Cell, Anaplastic | Details | |
| Anti-CD30 CAR-T cell therapy (National Cancer Institute) | Hu30-CD28z | Phase 1 Clinical | National Cancer Institute | Lymphoma, T-Cell, Peripheral; Enteropathy-Associated T-Cell Lymphoma; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Large-Cell, Anaplastic; Lymphoma | Details |
| PRA-052 | PRA-052 | Phase 1 Clinical | Prometheus Biosciences Inc | Colitis, Ulcerative | Details |
| SGN-CD30C | SGN-CD30C | Phase 1 Clinical | Seattle Genetics Inc | Lymphoma | Details |
| Anti-CD30 chimeric antigen receptor T cell therapy (Yake Biotechnology) | Clinical | Shanghai YaKe Biotechnology Co Ltd | Hodgkin Disease | Details |
This web search service is supported by Google Inc.







