
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































This protein carries no "tag".
The protein has a calculated MW of 14.1 kDa. The protein migrates as 15-16 kDa and 17-20 kDa under reducing (R) condition, and 30-40 kDa under non-reducing (NR) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
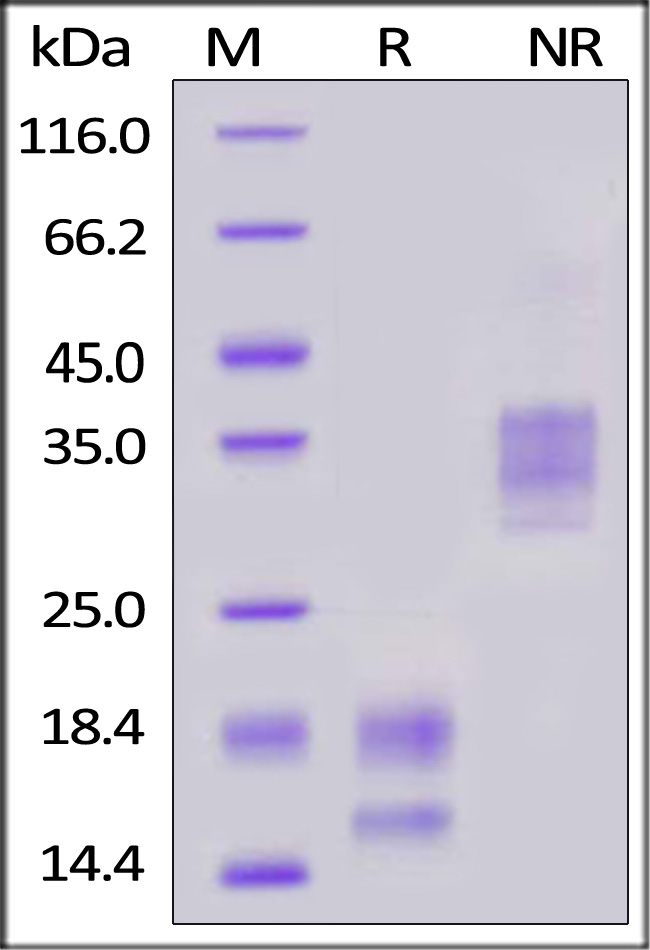
Human VEGF121 Protein, premium grade on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
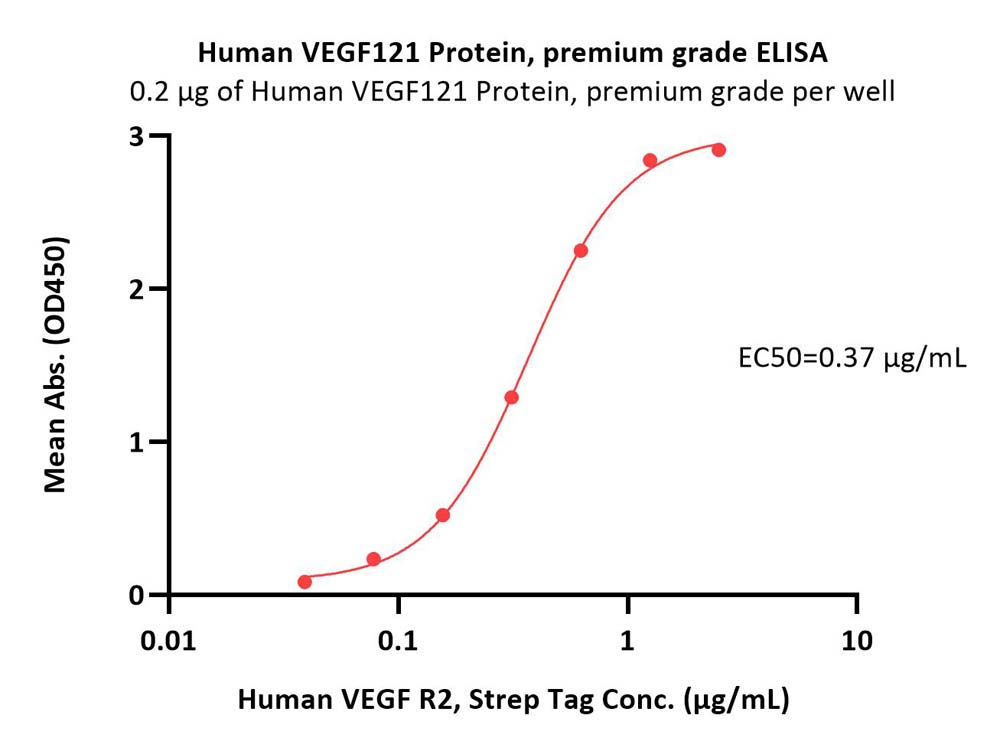
Immobilized Human VEGF121 Protein, premium grade (Cat. No. VE1-H4213) at 2 μg/mL (100 μL/well) can bind Human VEGF R2, Strep Tag (Cat. No. KDR-H5280) with a linear range of 0.15-2.5 μg/mL (QC tested).
Price(USD) : $205.00
Price(USD) : $350.00
Price(USD) : $2265.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab biosimilar (Qilu Pharma) | QL-1101 | Approved | Qilu Pharmaceutical Co Ltd | 安可达 | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Qilu Pharmaceutical Co Ltd | 2019-12-06 | Neoplasms; Colorectal Neoplasms; Carcinoma, Neuroendocrine; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Innovent Biologics) | IBI-305; IBI305; IBI 305; CHS-305 | Approved | Innovent Biologics(Suzhou) Co Ltd | 达攸同, BYVASDA, Bevagen | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Innovent Biologics(Suzhou) Co Ltd | 2020-06-17 | Ovarian Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Pfizer) | PF-6439535; PF-06439535 | Approved | Pfizer Inc | Zirabev | EU | Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Carcinoma, Renal Cell; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Pfizer Europe Ma Eeig | 2019-02-14 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Elea) | Approved | Laboratorio Elea Phoenix Sa | Lumiere | Argentina | Macular Degeneration | Laboratorio Elea Phoenix Sa | 2018-04-26 | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Samsung Bioepis) | SP-8; SB-8 | Approved | Samsung Bioepis Co Ltd | Aybintio | EU | Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Uterine Cervical Neoplasms; Carcinoma, Renal Cell; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Peritoneal Neoplasms | Samsung Bioepis Nl Bv | 2020-08-19 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ranibizumab biosimilar (Samsung Bioepis) | AM002; SB-11; SB11 | Approved | Samsung Bioepis Co Ltd | BYOOVIZ | EU | Macular Edema; Diabetic Retinopathy; Wet Macular Degeneration; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic macular oedema | Samsung Bioepis Nl Bv | 2021-08-18 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy; Retinal Vein Occlusion; Choroidal Neovascularization | Details |
| Bevacizumab biosimilar (Intas Pharmaceuticals) | INTP-24 | Approved | Intas Biopharmaceuticals | Bevatas | India | Carcinoma, Non-Small-Cell Lung; Breast Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Carcinoma, Renal Cell; Colorectal Neoplasms; Fallopian Tube Neoplasms | Intas Biopharmaceuticals | 2017-10-04 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Ranibizumab biosimilar (Formycon/Bioeq) | CHS-201; FYB-201 | Approved | Formycon AG, Bioeq Gmbh | CIMERLI, Ranivisio | United States | Macular Edema; Retinal Vein Occlusion; Choroidal Neovascularization; Macular Degeneration; Diabetic Retinopathy; Diabetic macular oedema; Wet Macular Degeneration | Coherus Biosciences Inc | 2022-08-02 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic Retinopathy; Macular Degeneration | Details |
| Bevacizumab biosimilar (AryoGen Biopharma) | BE1040V | Approved | Aryogen Biopharma | Stivant | Iran | Uterine Cervical Neoplasms; Metastatic breast cancer; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Glioblastoma | Aryogen Biopharma | 2019-06-01 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Metastatic breast cancer; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Shanghai Institute Of Biological Products) | SIBP-04 | Approved | Shanghai Institute Of Biological Products Co Ltd | 生唯宁 | Mainland China | Colorectal Neoplasms; Glioblastoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Ovarian Neoplasms | Shanghai Institute Of Biological Products Co Ltd | 2025-01-08 | Ovarian Neoplasms; Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Zydus Cadila) | Approved | Zydus Cadila | Bryxta | India | Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Colorectal Neoplasms; Glioblastoma; Metastatic breast cancer; Carcinoma, Renal Cell | Zydus Cadila | 2017-01-01 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details | |
| Bevacizumab biosimilar (TOT Biopharm) | TAB-008; TOT-102; TAB008; TOT102 | Approved | Tot Biopharm Co Ltd | 朴欣汀, Pusintin | Mainland China | Carcinoma, Non-Small-Cell Lung; Colorectal Neoplasms | Tot Biopharm Co Ltd | 2021-12-01 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab | G180CU; RO-4876646; RG-435; NSC-704865; G180CL; R-435; G180DL | Approved | Genentech Inc | 安维汀, Avastin | United States | Colorectal Neoplasms | Genentech Inc | 2004-02-26 | Liver Neoplasms; Infections; Respiratory Tract Infections; Pterygium; Ovarian Neoplasms; Solid tumours; Arteriovenous Malformations; Kidney Neoplasms; Head and Neck Neoplasms; Fibrosarcoma; Leiomyosarcoma; Epistaxis; Telangiectasia, Hereditary Hemorrhagic; Leukemia; HIV Infections; Leukemia, Myelogenous, Chronic; Ependymoma; Skin Melanoma; Meningeal Carcinomatosis; Telangiectasis; Diabetes Mellitus, Type 2; Macular Edema; Carcinoma, Renal Cell; Carcinoma, Basal Cell; Esthesioneuroblastoma, Olfactory; Carcinoid Tumor; Rectal Neoplasms; Carcinoma; Hemangioblastoma; Granuloma, Lethal Midline; Abdominal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Vaginal Neoplasms; Esophageal Neoplasms; Neoplasms, Glandular and Epithelial; Virus Diseases; Papillomavirus Infections; Neoplasms, Squamous Cell; Respiratory Tract Diseases; Glioblastoma; Carcinoma, Ovarian Epithelial; Neoplasms; Pancreatic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Carcinoma, Verrucous; Salivary Gland Neoplasms; Nose Neoplasms; Colonic Neopla | Details |
| Bevacizumab biosimilar(Dr. Reddy's Laboratories) | DRL-BZ | Approved | Dr.Reddy's Laboratories Ltd | Versavo, Persivia | India | Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Peritoneal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung; Carcinoma, Renal Cell | Dr.Reddy's Laboratories Ltd | 2019-08-19 | Ovarian Neoplasms; Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Celltrion) | CTP-16; CT-16; CT-P16 | Approved | Celltrion Inc | Vegzelma | EU | Ovarian Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Carcinoma, Renal Cell | Celltrion Healthcare Hungary Kft | 2022-08-17 | Ovarian Neoplasms; Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept biosimilar (Momenta/Mylan) | M-710; MYL-1701P | Approved | Momenta, Mylan Nv | YESAFILI | EU | Myopia, Degenerative; Diabetic Retinopathy; Retinal Vein Occlusion; Diabetes Complications; Macular Edema | Viatris Ltd | 2023-09-15 | Macular Edema; Myopia, Degenerative; Diabetic macular oedema; Wet Macular Degeneration; Diabetes Complications; Diabetic Retinopathy; Retinal Vein Occlusion | Details |
| Ranibizumab biosimilar (Intas Biopharmaceuticals) | Approved | Intas Biopharmaceuticals | Razumab | India | Macular Degeneration | Intas Biopharmaceuticals | 2015-01-01 | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Outlook) | ONS-5010; ONS-1045 | Approved | Outlook Therapeutics Inc | Lytenava | EU | Wet Macular Degeneration | Outlook Therapeutics Ltd, null | 2024-05-27 | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration; Retinal Vein Occlusion | Details |
| Ranibizumab biosimilar (Xbrane) | Approved | Xbrane Biopharma Ab | Ximluci | EU | Macular Edema; Wet Macular Degeneration; Diabetes Complications; Diabetic Retinopathy | Stada Arzneimittel Ag | 2022-11-09 | Macular Edema; Wet Macular Degeneration; Diabetes Complications; Macular Degeneration; Diabetic Retinopathy | Details | |
| Bevacizumab biosimilar (Boan Biopharma) | LY-01008; BA-1101 | Approved | Shandong Boan Biotechnology Co Ltd | 博优诺, Boyounuo | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Shandong Boan Biotechnology Co Ltd | 2021-05-07 | Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Shanghai Henlius Biotech) | HLX-04; HLX04-O | Approved | Shanghai Henlius Biotech Inc | 汉贝泰 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Shanghai Henlius Biopharmaceuticals Co Ltd | 2021-11-30 | Solid tumours; Carcinoma; Rectal Neoplasms; Carcinoma, Ovarian Epithelial; Glioblastoma; Wet Macular Degeneration; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Hepatocellular; Macular Degeneration; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Biocad) | BCD-021 | Approved | BIOCAD JSC | Avegra | Russian Federation | Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Glioblastoma; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial | BIOCAD JSC | 2015-11-25 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Wet Macular Degeneration; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Macular Degeneration | Details |
| Bevacizumab biosimilar (Sinocelltech) | SCT-510; SCT510A; SCT-510A | Approved | SinoCelltech Ltd | 安贝珠 | Mainland China | Glioblastoma; Peritoneal Neoplasms; Uterine Cervical Neoplasms; Ovarian Neoplasms; Fallopian Tube Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | SinoCelltech Ltd | 2023-06-27 | Ovarian Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Wet Macular Degeneration; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms; Macular Degeneration | Details |
| Ranibizumab biosimilar (Qilu Pharmaceutical) | BCD-300; QL-1205 | Approved | Biocnd, Qilu Pharmaceutical Co Ltd | Rimmyrah | EU | Myopia, Degenerative; Macular Edema; Wet Macular Degeneration; Choroidal Neovascularization; Diabetes Complications | Qilu Pharma Spain Sa | 2024-01-05 | Macular Edema; Myopia, Degenerative; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Macular Degeneration; Myopia; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic Retinopathy | Details |
| Bevacizumab biosimilar (Allergan/Amgen) | ABP-215 | Approved | Amgen Inc | Mvasi | United States | Uterine Cervical Neoplasms; Carcinoma, Renal Cell; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Glioblastoma | Amgen Inc | 2017-09-14 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept biosimilar (Qilu Pharmaceutical) | QL-1207 | Approved | Qilu Pharmaceutical Co Ltd | Mainland China | Macular Degeneration; Diabetic macular oedema | Qilu Pharmaceutical Co Ltd | 2023-12-13 | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details | |
| Ranibizumab biosimilar (Senju) | GBS-007; OT-701; SJP-0133 | Approved | Senju Pharmaceutical Co Ltd | Japan | Macular Degeneration | Senju Pharmaceutical Co Ltd | 2021-09-27 | Wet Macular Degeneration; Macular Degeneration | Details | |
| Aflibercept biosimilar (Amgen) | ABP-938 | Approved | Amgen Inc | PAVBLU | United States | Diabetic Retinopathy; Macular Edema; Wet Macular Degeneration; Diabetic macular oedema | Amgen Inc | 2024-08-23 | Macular Edema; Wet Macular Degeneration; Vascular Diseases; Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy | Details |
| Bevacizumab biosimilar (mAbixience) | MB02; BEVZ-92; BEVZ92-MB02; AP-01 | Approved | Mabxience Sa | Alymsys | Argentina | Colorectal Neoplasms; Carcinoma, Renal Cell; Peritoneal Neoplasms; Carcinoma, Non-Small-Cell Lung; Breast Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms | Stada Arzneimittel Ag | 2013-10-25 | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Glioblastoma; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Neovasculgen | PI-VEGF165 | Approved | Human Stem Cells Institute | Neovasculgen | Russian Federation | Peripheral Arterial Disease | Human Stem Cells Institute | 2011-12-07 | Peripheral Arterial Disease; Peripheral Nerve Injuries | Details |
| Nivolumab/Hyaluronidase | ONO-4538HSC | Approved | Bristol-Myers Squibb Company, Ono Pharmaceutical Co Ltd | OPDIVO QVANTIG, Opdivo Qvantig | United States | Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Transitional Cell; Esophageal Neoplasms; Colorectal Neoplasms; Melanoma; Carcinoma, Hepatocellular | Bristol-Myers Squibb Company | 2024-12-27 | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Melanoma; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (CTTQ Pharma) | TQ-B2302 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 安倍斯 | Mainland China | Glioblastoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2023-02-28 | Ovarian Neoplasms; Rectal Neoplasms; Glioblastoma; Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Biocon/Mylan) | MYL-1402O | Approved | Biocon Ltd | Lextemy, KRABEVA, Abevmy | India | Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Renal Cell; Colorectal Neoplasms; Ovarian Neoplasms; Brain Neoplasms | Mylan Pharmaceuticals Private Ltd | 2017-11-27 | Ovarian Neoplasms; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Brain Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Conbercept | FP-3; KH-902 | Approved | Chengdu Kanghong Biotechnologies Co Ltd | 朗沐, Langmu | Mainland China | Macular Degeneration | Chengdu Kanghong Biotechnologies Co Ltd | 2013-11-27 | Vision Disorders; Macular Edema; Diabetic macular oedema; Wet Macular Degeneration; Hemangioma; Retinoblastoma; Uveitis; Macular Degeneration; Diabetes Mellitus; Corneal Neovascularization; Retinal Vein Occlusion; Choroidal Neovascularization | Details |
| Bevacizumab biosimilar(Apotex ) | Approved | Apotex Inc | BAMBEVI | Canada | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Glioblastoma | Apotex Inc | 2021-09-23 | Carcinoma, Ovarian Epithelial; Glioblastoma; Colorectal Neoplasms; Peritoneal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Aflibercept | BAY-865321; BAT-86-5321 | Approved | Regeneron Pharmaceuticals Inc, Bayer AG | Eylea, 艾力雅 | United States | Macular Degeneration; Macular Edema | Regeneron Pharmaceuticals Inc | 2011-11-18 | Wet Macular Degeneration; Corneal Neovascularization; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Retinopathy of Prematurity; Retinal Vein Occlusion; Choroidal Neovascularization; Diabetic Retinopathy; Macular Degeneration; Lymphoma, Non-Hodgkin; Retinal Degeneration; Eye Diseases; Vitreous Hemorrhage; Diabetes Complications; Colorectal Neoplasms; Prostatic Neoplasms; Drug-Related Side Effects and Adverse Reactions; Diabetic macular oedema; Retinitis Pigmentosa; Multiple Myeloma; Myopia, Degenerative; Retinal Diseases; Central Serous Chorioretinopathy; Colonic Neoplasms; Neoplasms; Choroid Diseases; Rectal Neoplasms; Diabetes Mellitus, Type 2; Glaucoma, Neovascular; Macular Edema; Cataract; Diabetes Mellitus, Type 1 | Details |
| Bevacizumab biosimilar (Jiangsu Hengrui Medicine) | BP-102 | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妥 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Suzhou Suncadia Biopharmaceuticals Co Ltd | 2021-06-22 | Solid tumours; Triple Negative Breast Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Aflibercept biosimilar (HEXAL/Sandoz) | SOK-583A1; SOK583A1; SOK-583 | Approved | Sandoz, Hexal | ENZEEVU, Afqlir | United States | Wet Macular Degeneration | Sandoz Inc | 2024-08-09 | Retinal Diseases; Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Celltrion) | CT-P42 | Approved | Celltrion Inc | Eydenzelt | South Korea | Wet Macular Degeneration; Diabetic macular oedema | Celltrion Inc | 2024-05-30 | Wet Macular Degeneration; Diabetic macular oedema | Details |
| Ziv-aflibercept | BAY-865321; AVE-0005 | Approved | Regeneron Pharmaceuticals Inc, Sanofi | Zaltrap | United States | Colorectal Neoplasms | Sanofi-Aventis U.S. Llc | 2012-08-03 | Endometrial Neoplasms; Urethral Neoplasms; Breast Neoplasms; Brain Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Ureteral Neoplasms; Genital Neoplasms, Female; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Astrocytoma; Gliosarcoma; Lung Neoplasms; Lymphoma; Metastatic breast cancer; Prostatic Neoplasms; Carcinoma, Squamous Cell; Lymphoma, Non-Hodgkin; Thyroid Neoplasms; Glioma; Carcinoma, Neuroendocrine; Uterine Neoplasms; Fallopian Tube Neoplasms; Retinal Vein Occlusion; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Melanoma; Small Cell Lung Carcinoma; Skin Melanoma; Leukemia; Solid tumours; Multiple Endocrine Neoplasia Type 1; Leiomyosarcoma; Rectal Neoplasms; Carcinoma; Carcinoma, Renal Cell; Carcinoid Tumor; Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Papillary; Ovarian Neoplasms; Myelodysplastic Syndromes; Carcinoma, Transitional Cell; Leukemia, Myelomonocytic, Chronic; Glioblastoma; Carcinoma, Ovarian Epithelial; L | Details |
| Ranibizumab | RG-3645; rhu-Fab-VEG; AMD-rhuFab-V2; AMD-Fab; rhuFab-V2; Y-0317; RFB-002; RG-6321 | Approved | Novartis Pharma Ag, Genentech Inc | Lucentis, 诺适得, Susvimo | United States | Macular Degeneration | Genentech Inc | 2006-06-30 | Retinal Degeneration; Hemangioma; Retinoblastoma; Vascular Diseases; Retinal Detachment; Pseudoxanthoma Elasticum; Retinal Neovascularization; Cardiovascular Diseases; Diabetes Complications; Macular telangiectasia; Ischemia; Uveitis; Vitreous Hemorrhage; Eye Diseases; Glaucoma; Choroidal Neovascularization; Retinal Vein Occlusion; Diabetes Mellitus; Macular Degeneration; Diabetic Retinopathy; Myopia; Corneal Neovascularization; von Hippel-Lindau Disease; Conjunctival Neoplasms; Retinopathy of Prematurity; Port-Wine Stain; Melanoma; Macular telangiectasia 2; Strongyloidiasis; Optic Neuropathy, Ischemic; Polypoidal choroidal vasculopathy; Pterygium; Epistaxis; Telangiectasia, Hereditary Hemorrhagic; Retinal Telangiectasis; Cataract; Diabetic Angiopathies; Iris Diseases; Macular Edema; Depression; Telangiectasis; Vision Disorders; Vitreous Detachment; Glaucoma, Neovascular; Neovascularization, Pathologic; Central Serous Chorioretinopathy; Histoplasmosis; Myopia, Degenerative; Pathologic Processes; Retinal Disea | Details |
| Aflibercept biosimilar (Bioeq) | FYB-203 | Approved | Formycon AG | AHZANTIVE, Baiama | United States | Diabetic Retinopathy; Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Retinal Vein Occlusion; Macular Degeneration | Formycon AG | 2024-06-28 | Macular Edema; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy; Retinal Vein Occlusion | Details |
| Aflibercept biosimilar (Samsung Bioepis) | SB-15; AM003; SB15 | Approved | Samsung Bioepis Co Ltd | OPUVIZ, Opuviz, AFILIVU | United States | Diabetic macular oedema; Macular Edema; Wet Macular Degeneration; Diabetic Retinopathy | Samsung Bioepis Co Ltd | 2024-05-20 | Macular Edema; Diabetic macular oedema; Wet Macular Degeneration; Macular Degeneration; Diabetic Retinopathy | Details |
| Bevacizumab biosimilar (Reliance Life Sciences) | R-TPR-023 | Approved | Reliance Life Sciences | BevaciRel | India | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Carcinoma, Renal Cell; Uterine Cervical Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms | Reliance Life Sciences | 2016-06-13 | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Hualan Biological Engineering) | WBP-264 | Approved | Hualan Genetic Engineering Co Ltd | Mainland China | Peritoneal Neoplasms; Uterine Cervical Neoplasms; Colorectal Neoplasms; Glioblastoma; Carcinoma, Hepatocellular; Fallopian Tube Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Non-Small-Cell Lung | Hualan Genetic Engineering Co Ltd | 2024-11-15 | Carcinoma, Ovarian Epithelial; Glioblastoma; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details | |
| Faricimab | RO-6867461; RG-7716 | Approved | F. Hoffmann-La Roche Ltd, Genentech Inc | Vabysmo, 罗视佳 | United States | Wet Macular Degeneration; Diabetic macular oedema | Genentech Inc | 2022-01-28 | Polypoidal choroidal vasculopathy; Choroid Diseases; Macular Edema; Vision Disorders; Retinal Diseases; Wet Macular Degeneration; Diabetic macular oedema; Diabetes Complications; Choroidal Neovascularization; Myopia; Retinal Vein Occlusion; Macular Degeneration; Diabetes Mellitus; Diabetic Retinopathy | Details |
| Ivonescimab | SMT-112; AK-112 | Approved | Zhongshan Akeso Biopharma Co Ltd | 依达方 | Mainland China | Carcinoma, Non-Small-Cell Lung | Akeso-Sino Pharma Co Ltd | 2024-05-24 | Carcinoma, Neuroendocrine; Paget Disease, Extramammary; Sarcoma; Breast Neoplasms; Prostatic Neoplasms; Bile Duct Neoplasms; Genital Neoplasms, Female; Colorectal Neoplasms; Carcinoma, Squamous Cell; Endometrial Neoplasms; Carcinoma, Adenoid Cystic; Brain metastases; Penile Neoplasms; Angiomyolipoma; Paraganglioma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Neoplasm Metastasis; Esophageal Neoplasms; Rhabdomyosarcoma; Solid tumours; Kidney Neoplasms; Pheochromocytoma; Carcinoma; Carcinoma, Renal Cell; Carcinoma, Basal Cell; Urachal Cyst; Stomach Neoplasms; Ovarian Neoplasms; Neoplasms; Perivascular Epithelioid Cell Neoplasms; Small Cell Lung Carcinoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Salivary Gland Neoplasms; Glioblastoma | Details |
| Bevacizumab biosimilar (Hetero Drugs) | Approved | Hetero Drugs Ltd | Cizumab | India | Colorectal Neoplasms | Hetero Drugs Ltd | 2016-06-27 | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar (Bio-Thera Solutions) | BAT-1706; GP-2019 | Approved | Bio-Thera Solutions Ltd | 普贝希, AVZIVI | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Bio-Thera Solutions Ltd, BeOne Medicines Ltd | 2021-11-17 | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Neoplasms; Glioblastoma; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Uterine Cervical Neoplasms | Details |
| Bevacizumab biosimilar (Betta/Mabworks) | MIL-60 | Approved | Institute Of Basic Medicine, Chinese Academy Of Medical Sciences, Beijing Mabworks Biotech Co Ltd | 贝安汀 | Mainland China | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Betta Pharmaceuticals Co Ltd | 2021-11-24 | Carcinoma, Ovarian Epithelial; Glioblastoma; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Bevacizumab biosimilar(Jiangsu Aosaikang) | ASK-1202; AMD-B; AK-3008; ASK-B1202; ASKB1202 | Phase 3 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ranibizumab biosimilar (Reliance Life Sciences Group) | R-TPR-024 | Phase 3 Clinical | Reliance Life Sciences | Macular Degeneration | Details |
| TR-009 | NOV-1501; ABL001-ABL Bio; CTX-009; HD-B001A; ABL-001-ABL Bio; HDB001A; TR-009; ES-104 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Ampullary Carcinoma; Gallbladder Neoplasms | Details |
| Bevacizumab biosimilar (Huaota Biopharm/Shanghai Junshi Biosciences) | JS-501; HOT-1010 | Phase 3 Clinical | Shanghai Huaota Biopharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar (Prestige BioPharma/Hanwha Biologics) | HD-204 | Phase 3 Clinical | Hanwha Biologics | Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Aflibercept biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Macular Degeneration | Details | |
| Bevacizumab biosimilar (Alphamab/R-Pharm) | RPH-001 | Phase 3 Clinical | Suzhou Alphamab Co Ltd, R-Pharm | Colorectal Neoplasms | Details |
| Bevacizumab biosimilar(Zhejiang Teruisi Pharmaceutical) | TRS-003 | Phase 3 Clinical | Zhejiang Teruisi Pharmaceutical Inc | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Ranibizumab biosimilar (Chong Kun Dang Pharmaceutical) | CKD-701 | Phase 3 Clinical | Chong Kun Dang Pharmaceutical Corp | Macular Degeneration | Details |
| Ranibizumab biosimilar (Lupin) | LUBT-010 | Phase 3 Clinical | Lupin Ltd | Macular Degeneration | Details |
| Bevacizumab biosimilar (Genor Biopharma) | GB-222 | Phase 3 Clinical | Genor Biopharma Co Ltd | Brain Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| Muparfostat sodium | PI-88 | Phase 3 Clinical | Australian National University | Liver Neoplasms; Skin Melanoma; Solid tumours; Neoplasms; Prostatic Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar(Enzene Biosciences) | Phase 3 Clinical | Enzene Biosciences Ltd | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar (Curateq Biologics) | BP01; BP-01 | Phase 3 Clinical | CuraTeQ Biologics Pvt Ltd | Colonic Neoplasms | Details |
| Aflibercept biosimilar (Shilpa Biologicals) | SBDM-03 | Phase 3 Clinical | Shilpa Biologicals Pvt Ltd | Wet Macular Degeneration | Details |
| Bevacizumab biosimilar (Centus Biotherapeutics) | FKB-238 | Phase 3 Clinical | Fujifilm Kyowa Kirin Biologics Co Ltd | Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| Aflibercept Biosimilar(Rophibio) | RBS-001 | Phase 3 Clinical | Rophibio Inc | Wet Macular Degeneration; Macular Degeneration | Details |
| Bevacizumab biosimilar(Laboratorios Sophia) | PRO-169 | Phase 3 Clinical | Laboratorios Sophia Sa De Cv | Diabetic macular oedema | Details |
| Aflibercept Biosimilar (Alvotech Swiss) | AVT-06; AVT-29 | Phase 3 Clinical | Alvotech Swiss Ag | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept biosimilar (Mabwell) | 9-MW-0813; 9MW-0813; 9MW0813; 9-MW0813 | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Diabetic macular oedema | Details |
| Bevacizumab biosimilar (Beijing Science Sun/Beijing Lvzhu) | K-11 | Phase 3 Clinical | Beijing Lvzhu Biological Technology Co Ltd, Beijing Science Sun Pharmaceutical Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| PM-8002 | PM8002; PM-8002; BNT-327 | Phase 3 Clinical | Liver Neoplasms; Solid tumours; Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Neoplasms; Neuroendocrine Tumors; Mesothelioma; Breast Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details | |
| Aflibercept biosimilar (Sam Chun Dang Pharm) | SCD-411 | Phase 3 Clinical | Sam Chun Dang Pharm Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Varisacumab | R-84; GNR-011; AT-001-IBCG | Phase 3 Clinical | Peregrine, The University Of Texas Southwestern Medical Center | Glioblastoma; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AZD-8601 | mRNA-AZD-8601; AZD-8601 | Phase 2 Clinical | Astrazeneca Plc, Moderna Inc | Heart Failure | Details |
| MP-0250 | MP-0250 | Phase 2 Clinical | Molecular Partners Ag | Neoplasms; Multiple Myeloma; Carcinoma, Non-Small-Cell Lung | Details |
| ALS-L1023 | ALS-L1023 | Phase 2 Clinical | Angiolab Inc | Sleep Bruxism; Metabolic Dysfunction-Associated Steatotic Liver Disease; Metabolic Syndrome; Rosacea; Otitis Media with Effusion; Temporomandibular Joint Disorders; Obesity, Abdominal; Macular Degeneration | Details |
| Bevacizumab biosimilar (Eastern Biotech) | JY-028 | Phase 2 Clinical | Beijing Eastern Biotech Co Ltd | Wet Macular Degeneration; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Macular Degeneration | Details |
| ELGN-EYE | ELGN-EYE | Phase 2 Clinical | Elgan Pharma Ltd | Retinopathy of Prematurity | Details |
| SCT-501(National Cancer Institute) | SCT-501 | Phase 2 Clinical | National Cancer Institute | Kidney Neoplasms | Details |
| Y-400 | Y-400 | Phase 2 Clinical | Wuhan Yzy Biopharma Co Ltd | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| BI-836880 | BI-836880 | Phase 2 Clinical | Ablynx Nv, C.H. Boehringer Sohn Ag & Co. Kg | Squamous Cell Carcinoma of Head and Neck; Neoplasms; Wet Macular Degeneration; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| SYB-509 | SYB-509 | Phase 1 Clinical | Yuanda Shuyang Life Sciences (Chengdu) Co Ltd | Carcinoma, Transitional Cell; Carcinoma, Hepatocellular | Details |
| Vanucizumab | RG-7221; RO-5520985; B800Z06O8K (UNII code) | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms; Colorectal Neoplasms | Details |
| Bevacizumab biosimilar (North China Pharmaceutical) | MG-021 | Phase 1 Clinical | North China Pharmaceutical Company Ltd | Colorectal Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung | Details |
| Bevacizumab biosimilar(Guilin Sanjin) | Phase 1 Clinical | Guilin Sanjin Pharmaceutical Co Ltd | Macular Degeneration | Details | |
| Ranibizumab biosimilar (CJSC Generium) | GNR-067 | Phase 1 Clinical | Cjsc Generium | Macular Degeneration | Details |
| Bevacizumab biosimilar (Shanghai Kangdai) | Phase 1 Clinical | Shanghai Kanda Bio-Technology Co Ltd | Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Bevacizumab biosimilar (JHL Biotech) | JHL-1149 | Phase 1 Clinical | Eden Biologics Inc | Neoplasms | Details |
| Ziv-aflibercept biosimiliar (Boan Biopharma) | LY-01012; BA-1103 | Phase 1 Clinical | Shandong Boan Biotechnology Co Ltd | Colorectal Neoplasms | Details |
| Pasireotide long-acting formulation (Camurus) | CAM-4071 | Phase 1 Clinical | Camurus Ab | Endocrine System Diseases | Details |
| Bevacizumab biosimilar (Tanvex BioPharma) | TX-16 | Phase 1 Clinical | Tanvex Biopharma | Colorectal Neoplasms | Details |
| PB-101 | PB-101 | Phase 1 Clinical | Panolos Bioscience Inc | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| IBI-333 | IBI-333 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Wet Macular Degeneration; Macular Degeneration | Details |
| IBI-324 | IBI-324 | Phase 1 Clinical | Innovent Biologics (Usa), Inc | Diabetic macular oedema | Details |
| EB-105 | EB-105 | Phase 1 Clinical | Eluminex Biosciences (Suzhou) Ltd | Diabetic macular oedema; Diabetic Retinopathy | Details |
| JS-207 | JS-207 | Phase 1 Clinical | Shanghai Junshi Biological Engineering Co Ltd | Neoplasms | Details |
| Y-332 | Y-332 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd, Wuhan Yzy Medical Science And Technology Co Ltd | Solid tumours | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
| Aflibercept biosimilar(Generium) | GNR-098 | Phase 1 Clinical | Generium Pharmaceuticals | Macular Degeneration | Details |
| CS-2009 | CS-2009 | Phase 1 Clinical | Cstone Pharmaceuticals | Solid tumours; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar (Gedeon Richter) | Phase 1 Clinical | Gedeon Richter Plc | Neoplasms | Details | |
| HG-202 | HG-202; HG202 | Phase 1 Clinical | HuiGene Therapeutics Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Zein Bioteccnology) | Phase 1 Clinical | Zein Bioteccnology Co Ltd | Diabetic macular oedema | Details | |
| VEGFA-targeting Gene Therapy(BDgene) | BD311 | Phase 1 Clinical | Shanghai BDgene Technology Co Ltd | Diabetic macular oedema; Macular Degeneration; Retinal Vein Occlusion | Details |
| ASKG-712 | ASKG-712; AM-712 | Phase 1 Clinical | Askgene Pharma | Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| OB-318 | OB-318 | Phase 1 Clinical | Oneness Biotech Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| Bevacizumab biosimilar(Guangdong Dongyangguang) | Phase 1 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Carcinoma, Non-Small-Cell Lung | Details | |
| Colorectal cancer vaccine (Immunovo/Pepscan Therapeutics) | Phase 1 Clinical | Pepscan Systems | Colorectal Neoplasms | Details | |
| Bevacizumab biosimilar(Bioxpress) | BXT-2316 | Clinical | Bioxpress Therapeutics Sa | Carcinoma, Renal Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.







