
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































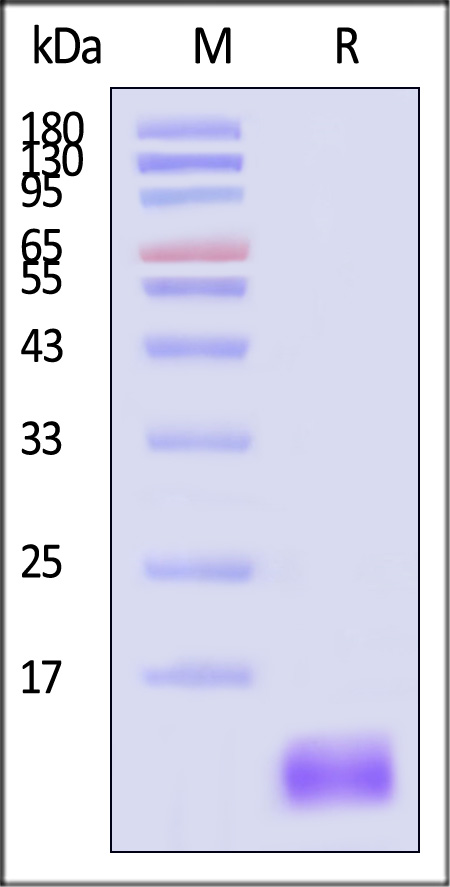
This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 7.9 kDa. The protein migrates as 13-14 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>90% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
Cynomolgus / Rhesus macaque BCMA, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
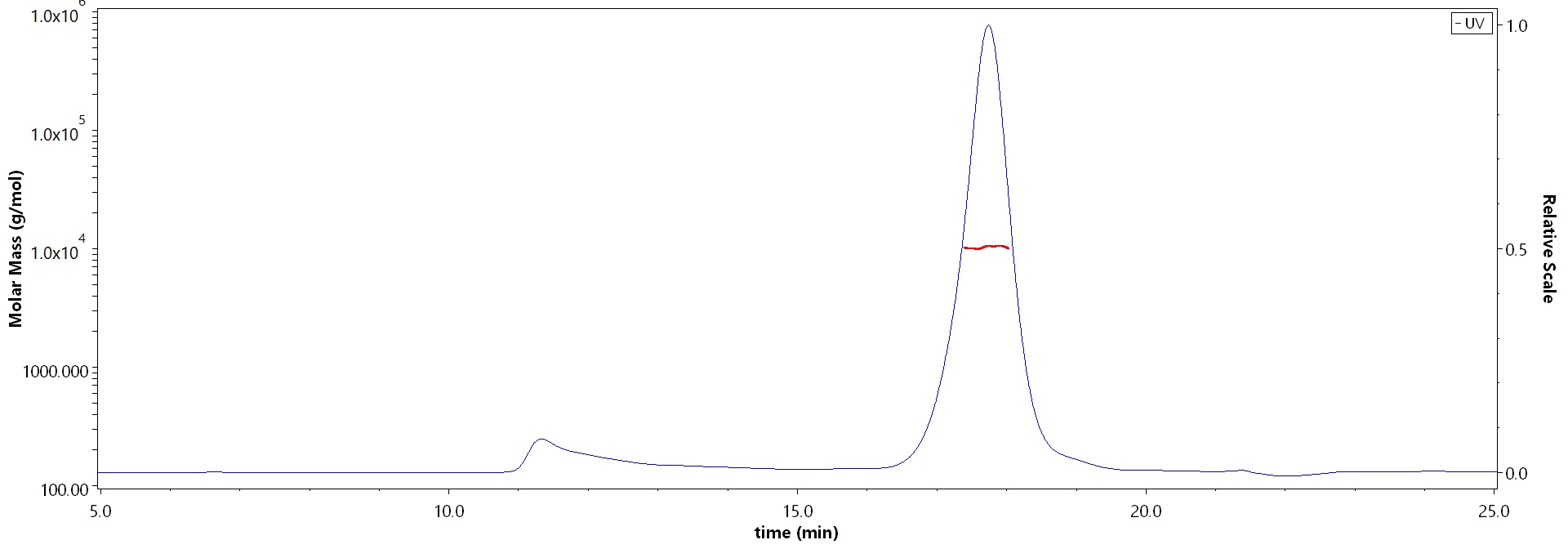
The purity of Cynomolgus / Rhesus macaque BCMA, His Tag (Cat. No. BCA-C52H7) is more than 85% and the molecular weight of this protein is around 10-20 kDa verified by SEC-MALS.
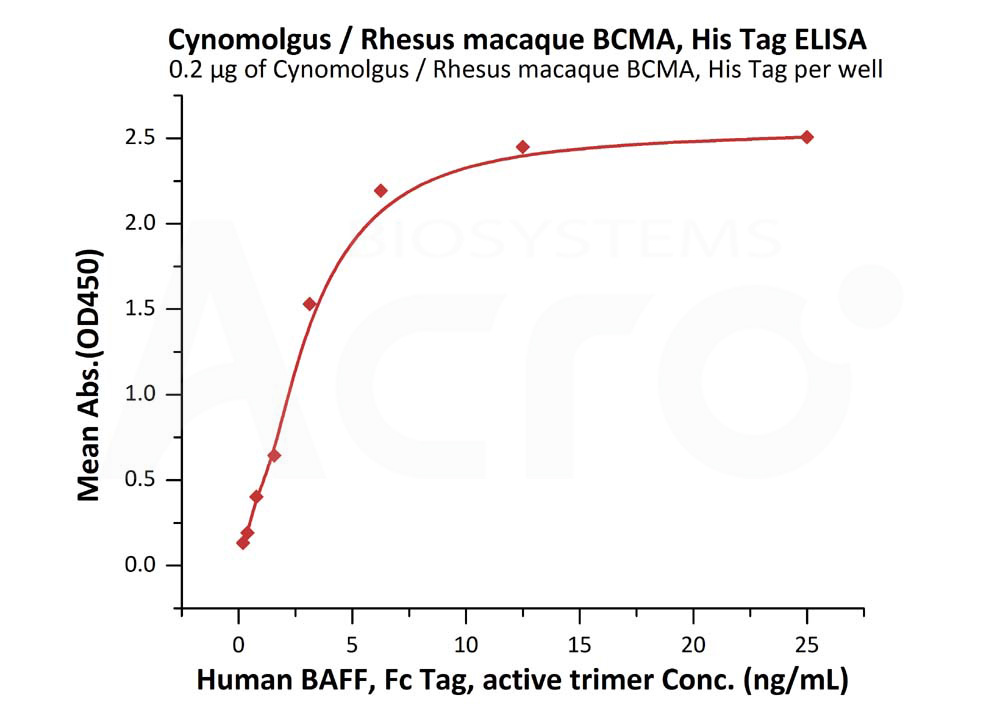
Immobilized Cynomolgus / Rhesus macaque BCMA, His Tag (Cat. No. BCA-C52H7) at 2 μg/mL (100 μL/well) can bind Human BAFF, Fc Tag, active trimer (Cat. No. BAF-H5261) with a linear range of 0.2-3 ng/mL (QC tested).

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Belantamab mafodotin | GSK-2857916; GSK2857916 | Approved | Glaxosmithkline Plc | BLENREP | Mainland China(BOAO LECHENG) | Multiple Myeloma | Glaxosmithkline Plc | 2020-08-05 | Bone Marrow Neoplasms; Lymphoma, B-Cell; Hematologic Diseases; Paraproteinemias; Immunoglobulin Light-chain Amyloidosis; Plasmacytoma; Corneal Diseases; Neoplasms; Blood Protein Disorders; Multiple Myeloma; Plasmablastic Lymphoma; Neoplasms, Plasma Cell; Amyloidosis | Details |
| Idecabtagene vicleucel | bb-2121 | Approved | Bluebird Bio Inc | Abecma | United States | Multiple Myeloma | Celgene Corp | 2021-03-26 | Multiple Myeloma | Details |
| Zevorcabtagene Autoleucel | CT-053-CARsgen | Approved | Carsgen Biomedicine (Shanghai) Co Ltd | 赛恺泽 | Mainland China | Multiple Myeloma | Kaixing Life Technology (Shanghai) Co Ltd | 2024-02-23 | Multiple Myeloma | Details |
| Equecabtagene Autoleucel | IBI-326; CT-103A; CT103A | Approved | Nanjing Iaso Biotherapeutics Co Ltd, Innovent Biologics(Suzhou) Co Ltd | 福可苏, FUCASO | Mainland China | Multiple Myeloma | Nanjing Xunlu Biomedicine Co Ltd | 2023-06-30 | Myasthenia Gravis; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Myositis; Autoimmune Diseases; Multiple Sclerosis; Multiple Myeloma; Lupus Nephritis; Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease; Neuromyelitis Optica; Lupus Erythematosus, Systemic; POEMS Syndrome; Autoimmune Diseases of the Nervous System; Encephalitis | Details |
| Teclistamab | JNJ-64007957; JNJ-64007959; JNJ-7957 | Approved | Genmab A/S, Johnson & Johnson Innovative Medicine | TECAYLI, TECVAYLI, 泰立珂 | EU | Multiple Myeloma | Janssen-Cilag International Nv | 2022-08-23 | Hematologic Diseases; Immunoglobulin Light-chain Amyloidosis; Smoldering Multiple Myeloma; Multiple Myeloma | Details |
| Ciltacabtagene autoleucel | JNJ-4528; LCAR-B38M; JNJ-68284528 | Approved | Nanjing Legend Biotechnology Co Ltd | Carvykti, 卡卫荻 | United States | Multiple Myeloma | Nanjing Legend Biotechnology Co Ltd, Janssen Research & Development Llc | 2022-02-28 | Multiple Myeloma | Details |
| Elranatamab | PF-06863135; PF‑3135; RN-613 | Approved | Pfizer Inc | Elrexfio, ELREXFIO, ELREXFIO™ | United States | Multiple Myeloma | Pfizer Inc | 2023-08-14 | Bone Marrow Neoplasms; Smoldering Multiple Myeloma; Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Autologous BCMA-directed CAR T-cell therapy (The First Affiliated Hospital of Soochow University) | Phase 3 Clinical | The First Affiliated Hospital Of Soochow University | Leukemia; Multiple Myeloma | Details | |
| Anitocabtagene autoleucel | CART-ddBCMA; Kite-772 | Phase 3 Clinical | Arcellx Inc | Myasthenia Gravis; Neuromuscular Manifestations; Autoimmune Diseases; Multiple Myeloma; Muscular Diseases; Muscle Weakness; Autoimmune Diseases of the Nervous System | Details |
| CD3 and B-cell maturation antigen T-cell engaging bispecific antibody (AbbVie/TeneoOne) | ABBV-383; TNB-383B | Phase 3 Clinical | TeneoOne Inc | Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma | Details |
| Anti-BCMA chimeric antigen receptor T cell therapy (Cartesian) | Descartes-08 | Phase 3 Clinical | Cartesian Therapeutics Inc | Myasthenia Gravis; Multiple Myeloma; Lupus Erythematosus, Systemic | Details |
| BCMA CAR-T Cells (Pregene) | PRG-1801; GLPG-5301; DRL-1801 | Phase 2 Clinical | Purpura, Thrombocytopenic, Idiopathic; Immunoglobulin G4-Related Disease; Multiple Myeloma; Lupus Nephritis | Details | |
| Anti BCMA CAR T cell therapy (Shanghai Hrain Biotechnology) | BCMA-CART; HR003 | Phase 2 Clinical | Hrain Biotechnology Co Ltd | Multiple Myeloma | Details |
| Anti BCMA chimeric antigen receptor T cell therapy (Chongqing Precision Biotech) | Phase 2 Clinical | Chongqing Precision Biotechnology Co Ltd | Lymphoma, B-Cell; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details | |
| BCMA CAR-T cell therapy (Chongqing Precision Biotech) | Phase 2 Clinical | Chongqing Precision Biotechnology Co Ltd | Plasmacytoma; Multiple Myeloma; Neoplasms, Plasma Cell | Details | |
| C-CAR088 | C-CAR088 | Phase 2 Clinical | Cellular Biomedicine (Shanghai) Co Ltd | Multiple Myeloma | Details |
| Orvacabtagene autoleucel | ET-140; FCARH-143; ET140-CAR; MCARH-171; JCARH-125 | Phase 2 Clinical | Juno Therapeutics Inc, Memorial Sloan Kettering Cancer Center, Eureka Therapeutics Inc | Multiple Myeloma | Details |
| GC-012F | GC-012F; GC012F; AZD-0120; AZD0120 | Phase 2 Clinical | Gracell Biotechnologies (Shanghai) Co Ltd | Myasthenia Gravis; Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin | Details |
| BCMA-PD1 CAR T cell therapy (General Hospital of the People's Liberation Army) | Phase 2 Clinical | People'S Liberation Army General Hospital Military Service | Multiple Myeloma | Details | |
| KQ-2003 | KQ2003; KQ-2003 | Phase 2 Clinical | Shanghai Keqi Pharmaceutical Technology Co Ltd | Solid tumours; Multiple Myeloma; POEMS Syndrome | Details |
| HDP-101 | HDP-101; HDP101-ATAC | Phase 2 Clinical | Heidelberg | Hematologic Diseases; Paraproteinemias; Multiple Myeloma | Details |
| REGN-5459 | REGN-5459 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Multiple Myeloma; Renal Insufficiency, Chronic | Details |
| BCMA/CD19 chimeric antigen receptor (CAR)-modified T cells Therapy(Peking University People's Hospital) | Phase 2 Clinical | Peking University People'S Hospital | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Myositis; Immunoglobulin G4-Related Disease; Sjogren's Syndrome; Scleroderma, Systemic; Antiphospholipid Syndrome; Lupus Erythematosus, Systemic; Acquired thrombotic thrombocytopenic purpura; Behcet Syndrome | Details | |
| anti-BCMA/FcRL5 CAR T cell therapy (Xuzhou Medical University) | Phase 2 Clinical | Xuzhou Medical University (Xzmu) | Multiple Myeloma | Details | |
| BCMA-GPRC5D CAR-T cells Therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Multiple Myeloma | Details | |
| Chimeric Antigen Receptor Modified T Cells Targeting BCMA(The First Affiliated Hospital of Xiamen University) | Phase 2 Clinical | The First Affiliated Hospital Of Xiamen University | Hematologic Neoplasms; Multiple Myeloma | Details | |
| BCD-248 | BCD-248 | Phase 2 Clinical | BIOCAD JSC | Multiple Myeloma | Details |
| Nanobody-based biepitope BCMA-targeting CAR-T cells therapy(Tahoe Chunyu Biotechnology) | Phase 2 Clinical | Hebei Tahoe Chunyu Biotechnology Co Ltd | Multiple Myeloma | Details | |
| CAR-T Cell Therapy(Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute, The No.2 Clinical Hospital of the Ministry of Health | Plasmacytoma; Multiple Myeloma | Details | |
| Fourth-gen CAR T Cells Targeting BCMA/CD19 therapy(Essen Biotech) | Phase 2 Clinical | Essen Biotech | Granulomatosis with Polyangiitis; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Microscopic Polyangiitis; Myositis; Scleroderma, Systemic; Autoimmune Diseases; Sjogren's Syndrome; Lupus Nephritis; Lupus Erythematosus, Systemic | Details | |
| Anti-BCMA CAR-NK Therapy(Shahid Beheshti University of Medical Sciences) | Phase 2 Clinical | Shahid Beheshti University Of Medical Sciences | Multiple Myeloma | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| BCMA-GPRC5D CAR-T Cells Therapy (Bio-Gene) | Phase 2 Clinical | Guangzhou Bio-Gene Technology Co Ltd | Multiple Myeloma | Details | |
| CART-ASCT-CART2 cells Therapy(Institute Of Hematology & Blood Diseases Hospital) | Phase 2 Clinical | Institute Of Hematology & Blood Diseases Hospital | Multiple Myeloma | Details | |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| SAR445514 | SAR445514; SAR-445514; IPH6401/SAR514 | Phase 2 Clinical | Sanofi | Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma | Details |
| CART-BCMA(Simnova) | SNC-102(Simnova); SNC102(Simnova) | Phase 2 Clinical | Shanghai Simnova Biotechnology Co Ltd | Multiple Myeloma | Details |
| Autologous anti-BCMA/GPRC5D CAR-T cell therapy (Shanghai YaKe Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma | Details | |
| CAR-BCMA(Sheba Medical Center) | Phase 2 Clinical | Sheba Medical Center, Israel | Multiple Myeloma | Details | |
| Humanized CART Directed Against BCMA | ARI-0002h | Phase 2 Clinical | Instituto De Salud Carlos Iii | Multiple Myeloma | Details |
| Descartes-11 | Descartes-11; Descartes-011 | Phase 2 Clinical | Cartesian Therapeutics Inc | Multiple Myeloma | Details |
| BCMA CAR-T Cell Therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Multiple Myeloma | Details | |
| GR-1803 | GR-1803 | Phase 2 Clinical | Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd, Genrix (Shanghai) Biopharmaceutical Co Ltd | Multiple Myeloma | Details |
| CM-336(Connaught Biomedical Technology) | CM-336; OM336 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Multiple Myeloma | Details |
| PHE-885 | PHE-885 | Phase 2 Clinical | Novartis Pharma Ag | Hematologic Neoplasms; Multiple Myeloma | Details |
| EMB-06 | EMB-06; EMB06; CN106; CND106; CND-106 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Multiple Myeloma; Pemphigus | Details |
| NXC-201 | HBI-0101; NXC-201 | Phase 2 Clinical | Immix Biopharma Inc, Hadassah Medical Organization | Immunoglobulin Light-chain Amyloidosis; Multiple Myeloma | Details |
| BCMA Targeted CAR-T Cell Therapy (Yake Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Neoplasms; Multiple Myeloma | Details | |
| BCMA TriTAC | HPN-217 | Phase 1 Clinical | Harpoon Therapeutics, Abbvie Inc | Multiple Myeloma | Details |
| ACTR 087/SEA BCMA combination therapy | Phase 1 Clinical | Unum Therapeutics Inc | Multiple Myeloma | Details | |
| bb-21217 | bb-21217 | Phase 1 Clinical | Bluebird Bio Inc, Bristol-Myers Squibb Company | Multiple Myeloma | Details |
| AMG-224 | AMG-224 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| Ispectamab debotansine | CC-99712; BMS-986352; SP-8919 | Phase 1 Clinical | Celgene Corp | Multiple Myeloma | Details |
| Pacanalotamab | AMG-420; BI-836909 | Phase 1 Clinical | Micromet Inc | Multiple Myeloma | Details |
| ALLO-715 | ALLO-715 | Phase 1 Clinical | Cellectis Sa | Multiple Myeloma | Details |
| Alnuctamab | CC-93269; EM-901; BMS-986349 | Phase 1 Clinical | Engmab Ag | Multiple Myeloma | Details |
| Pavurutamab | AMG-701 | Phase 1 Clinical | Amgen Inc | Multiple Myeloma | Details |
| CART-BCMA | MTV-273; CART-BCMA | Phase 1 Clinical | University Of Pennsylvania | Rejection of renal transplantation; Renal Insufficiency; Multiple Myeloma; Kidney Failure, Chronic | Details |
| BCMA-CD19 cCAR T cell therapy (iCell Gene Therapeutics) | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Multiple Myeloma; Lupus Erythematosus, Systemic; Pancytopenia; Waldenstrom Macroglobulinemia | Details | |
| Anti-BCMA anti-CD38 bispecific chimeric antigen receptor T cell therapy (Shengyan Pharmaceutical Technology) | Phase 1 Clinical | Shengyan Pharmaceutical Technology | Multiple Myeloma | Details | |
| Descartes-15 | Descartes-015; Descartes-15 | Phase 1 Clinical | Cartesian Therapeutics Inc | Solid tumours; Multiple Myeloma; Autoimmune Diseases of the Nervous System | Details |
| ACLX-001 | ACLX-001 | Phase 1 Clinical | Arcellx Inc | Multiple Myeloma | Details |
| CB-011 | CB-011 | Phase 1 Clinical | Caribou Biosciences Inc | Multiple Myeloma | Details |
| FT-576 | FT-576 | Phase 1 Clinical | University Of Minnesota, Fate Therapeutics Inc | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| P-BCMA-ALLO1 | P-BCMA-ALLO1; RG-6538 | Phase 1 Clinical | Janssen Biotech Inc, Transposagen Biopharmaceuticals Inc | Multiple Myeloma | Details |
| Anti-CD19/BCMA CAR NK cells therapy(The Children's Hospital of Zhejiang University School of Medicine) | KN5601; KN-5601 | Phase 1 Clinical | The Children'S Hospital, Zhejiang University School Of Medicine | Nephrosis; Autoimmune Diseases; Lupus Erythematosus, Systemic | Details |
| RD06-05 CART Cell Therapy(Nanjing Bioheng Biotech) | RD06-05 | Phase 1 Clinical | Nanjing Bioheng Biotech Co Ltd | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica; Lupus Erythematosus, Systemic | Details |
| Universal allogeneic anti-CD19/BCMA CAR T-cells therapy(XiNiao Biotechnology) | Phase 1 Clinical | Hangzhou XiNiao Biotechnology Co Ltd | Anemia, Hemolytic, Autoimmune | Details | |
| 68Ga-BC1 | 68Ga-BC1 | Phase 1 Clinical | The First Affiliated Hospital Of Beijing University | Multiple Myeloma; Neoplasms, Plasma Cell | Details |
| ESO-T01 | ESO-T01 | Phase 1 Clinical | Shenzhen Pregene Biopharma Co Ltd | Multiple Myeloma | Details |
| Universal BCMA-Targeted CAR-T Therapy(Wondercel) | REVO-UWD-00B | Phase 1 Clinical | Wondercel Therapeutics Co Ltd | Multiple Myeloma | Details |
| Anti-BCMA/CD70 CAR-T cells therapy(The Children'S Hospital, Zhejiang University School Of Medicine) | Phase 1 Clinical | The Children'S Hospital, Zhejiang University School Of Medicine | Nephrosis | Details | |
| PRG-2311 | PRG-2311 | Phase 1 Clinical | Immunoglobulin G4-Related Disease; Lupus Nephritis | Details | |
| BZE-2204 | BZE2204; BZE-2204 | Phase 1 Clinical | Shanghai Cell Therapy Group Co Ltd | Lymphoma, B-Cell; Multiple Myeloma | Details |
| OL-101 | OL-101 | Phase 1 Clinical | Overland Pharmaceuticals (Shanghai) Co Ltd | Multiple Myeloma | Details |
| Anti-CD19/BCMA CAR T-cell therapy(YaKe Biotechnology) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd, Zhejiang University | Lupus Nephritis; Lupus Erythematosus, Systemic | Details | |
| Zirconium-89 belantamab | 89Zr-GSK2857914 | Phase 1 Clinical | Glaxosmithkline Plc | Neoplasms; Multiple Myeloma | Details |
| RD-140 | RD140 | Phase 1 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| BCMA CAR-NK (Hrain Biotechnology) | HR012 | Phase 1 Clinical | Hrain Biotechnology Co Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| CD19/BCMA Hi-TCR-T cell therapy(Wuhan Union Hospital) | Phase 1 Clinical | Wuhan Union Hospital | Lupus Erythematosus, Systemic | Details | |
| SYS-6020 | SYS6020; SYS-6020 | Phase 1 Clinical | CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co Ltd | Myasthenia Gravis; Multiple Myeloma; Lupus Erythematosus, Systemic | Details |
| The autologous dual target BCMA/CD19-CAR-T cell therapy(Nanjing University School Of Medicine) | FKC-288; FKC288 | Phase 1 Clinical | Nanjing University School Of Medicine | Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Immunoglobulin G4-Related Disease; Lupus Nephritis | Details |
| YKST-02 | YKST02; YKST-02 | Phase 1 Clinical | Excyte Biopharma Ltd | Multiple Myeloma | Details |
| C-CAR168 CAR T-cell therapy (AbelZeta) | C-CAR168 | Phase 1 Clinical | Cellular Biomedicine (Shanghai) Co Ltd | Myasthenia Gravis; Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Lupus Erythematosus, Systemic; Neuromyelitis Optica; Muscular Diseases | Details |
| BCMAxGPRC5D CAR-T therapy (Juno Therapeutics) | BMS-986453 | Phase 1 Clinical | Juno Therapeutics Inc | Multiple Myeloma | Details |
| SIM-0500 | SIM0500; SIM-0500 | Phase 1 Clinical | Simcere Pharmaceutical Group Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| Recombinant anti BCMA/CD3 bispecific antibody(Hualan Genetic Engineering) | Phase 1 Clinical | Hualan Genetic Engineering (Henan) Co Ltd | Multiple Myeloma | Details | |
| BCMA Targeted CAR-T Cell Therapy (Carbiogene Therapeutics) | CBG-002 CAR-T | Phase 1 Clinical | Carbiogene Therapeutics Co Ltd | Multiple Myeloma | Details |
| BCMA-CD19 CAR T cell therapy (Hunan Siweikang Pharma) | SWK001; SWK-001 | Phase 1 Clinical | Hunan Siweikang Pharmaceutical Co Ltd | Multiple Myeloma | Details |
| Anti-BCMA CAR T (Nanjing Kati Medical Technology) | Phase 1 Clinical | Nanjing Kati Medical Technology Co Ltd | Multiple Myeloma | Details | |
| Belantamab | GSK2857914; GSK-2857914 | Phase 1 Clinical | Glaxosmithkline Plc | Multiple Myeloma; Lupus Erythematosus, Systemic | Details |
| Anti-BCMA CAR T-cell therapy (Actavis/Eugia Pharma) | Phase 1 Clinical | Eugia Pharma Specialities Ltd, Actavis Inc | Multiple Myeloma | Details | |
| LUCAR-B68 cells Therapy(Nanjing Legend Biotech Co) | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Multiple Myeloma | Details | |
| Universal BCMA-CD19 CART cell therapy(BRL Medicine) | BRL-302 | Phase 1 Clinical | BRL Medicine Inc | Myasthenia Gravis; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Solid tumours; Autoimmune Diseases; Multiple Sclerosis; Lupus Nephritis; Neuromyelitis Optica; Prostatic Neoplasms | Details |
| TQB-2934 | TQB2934; TQB-2934 | Phase 1 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Multiple Myeloma | Details |
| MCARH-125 | MCARH-125 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Multiple Myeloma | Details |
| ISB-2001 | ISB-2001 | Phase 1 Clinical | Ichnos Sciences Sa | Multiple Myeloma | Details |
| OriC-321 | OriC-321; Ori-C-321 | Phase 1 Clinical | Zhejiang University, OriCell Therapeutics Co Ltd | Multiple Myeloma | Details |
| HY-027 | HY-027 | Phase 1 Clinical | Juventas Cell Therapy Ltd | Multiple Myeloma | Details |
| MEDI-2228 | MEDI-2228 | Phase 1 Clinical | Medimmune Llc | Multiple Myeloma | Details |
| IBI-346 | IBI-346 | Phase 1 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| BCMA-NKE | BCMA-NKE | Phase 1 Clinical | Bristol-Myers Squibb Company | Multiple Myeloma | Details |
| MCM-998 | MCM-998; LXG-250 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| BCMA-UCART cell therapy (Shanghai Bioray Laboratory) | Phase 1 Clinical | BRL Medicine Inc | Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Myasthenia Gravis; Multiple Sclerosis; Multiple Myeloma; Neuromyelitis Optica | Details | |
| ALLO-605 | ALLO-605; ALLO605 | Phase 1 Clinical | Cellectis Sa, Overland Pharmaceuticals (Shanghai) Co Ltd | Multiple Myeloma | Details |
| AUTO-8 | AUTO-8; AUTO8 | Phase 1 Clinical | Autolus Therapeutics Plc, University College London | Multiple Myeloma | Details |
| Recombinant humanized anti-BCMA/CD3 bispecific antibody(New Time Pharmaceutical) | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| CXCR4 modified anti-BCMA CAR T cell therapy (Sichuan University) | Phase 1 Clinical | Sichuan University | Multiple Myeloma | Details | |
| JWCAR129 | JWCAR129; JWCAR-129 | Phase 1 Clinical | Suzhou Yaomingjunuo Biotechnology Co Ltd | Multiple Myeloma | Details |
| Anti-BCMA CAR-T cell therapy (Xinqiao Hospital) | Phase 1 Clinical | Chongqing Xinqiao Hospital | Multiple Myeloma | Details | |
| BCMA-targeted universal LCAR-BCX cell therapy (Nanjing Legend Biotech) | LCAR-BCX | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Multiple Myeloma | Details |
| CD19/BCMA-targeted CAR-T Cell Therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University, Shanghai YaKe Biotechnology Co Ltd | Nephritis; Scleroderma, Systemic; Autoimmune Diseases; Sjogren's Syndrome; Multiple Myeloma; Lupus Nephritis; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| C-4-29 | Phase 1 Clinical | Chongqing Precision Biotechnology Co Ltd | Carcinoma, Renal Cell; Multiple Myeloma | Details | |
| Anti-BCMA CAR-T cell therapy (PersonGen Biomedicine) | Phase 1 Clinical | Persongen Biotherapeutics | Multiple Myeloma | Details | |
| BCMA CAR-T cell therapy (Hebei Senlang Biotechnology) | Phase 1 Clinical | Hebei Senlang Biological Technology Co Ltd | Multiple Myeloma | Details | |
| CC-98633 | CC-98633 | Phase 1 Clinical | Celgene Corp | Multiple Myeloma | Details |
| Anti-BCMA CAR T cell therapy (Wuhan Bio-Raid Biotechnology) | Clinical | Wuhan BioRaid Biotechnology Co Ltd | Hematologic Neoplasms | Details | |
| Anti-BCMA CAR T-cell therapy (Huazhong University of Science and Technology) | Clinical | Huazhong University Of Science And Technology | Multiple Myeloma | Details | |
| SA-102 | SA-102; SA102 | Clinical | Wuhan Si'an Medical Technology Co Ltd | Multiple Myeloma | Details |
| Allogeneic Anti-BCMA/GPRC5D Bispecific CAR-NK Cells therapy(Acellytron Therapeutics) | ACT-001(Acellytron Therapeutics) | Clinical | Acellytron Therapeutics | Multiple Myeloma | Details |
| Anti-BCMA chimeric antigen receptor natural killer cell therapy (Hangzhou Cheetah Cell Therapeutics) | Clinical | Multiple Myeloma | Details | ||
| PD-1Ab21-BCMACAR-T (CD biopharma) | CD-003; CD-203 | Clinical | Multiple Myeloma | Details | |
| BCMA targeting UCAR-T cell therapy (PersonGen BioTherapeutics) | UTAA17 | Clinical | Persongen Biotherapeutics (Suzhou) Co Ltd | Multiple Myeloma | Details |
| CT-0590(The First Affiliated Hospital Of Soochow University) | CT-0590 | Clinical | CARsgen Therapeutics Holdings Ltd | Multiple Myeloma | Details |
| UF-KURE-BCMA CAR-T Cells therapy(University Hospitals Cleveland Medical Center) | University Hospitals Cleveland Medical Center | Details |
This web search service is supported by Google Inc.







