
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































This protein carries a polyhistidine tag at the N-terminus.
The protein has a calculated MW of 81.4 KDa. The protein migrates as 85-115 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
FITC
Excitation source: 488 nm spectral line, argon-ion laser
Excitation Wavelength: 488 nm
Emission Wavelength: 535 nm
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in 25 mM MES, 500 mM NaCl, pH6.5 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please protect from light and avoid repeated freeze-thaw cycles.
This product is stable after storage at:

FITC-Labeled Human PSMA, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.

Immobilized Monoclonal Anti-Human PSMA Antibody, Human IgG1 at 5 μg/mL (100 μL/well) can bind FITC-Labeled Human PSMA, His Tag (Cat. No. PSA-HF244) with a linear range of 0.005-0.16 μg/mL (QC tested).
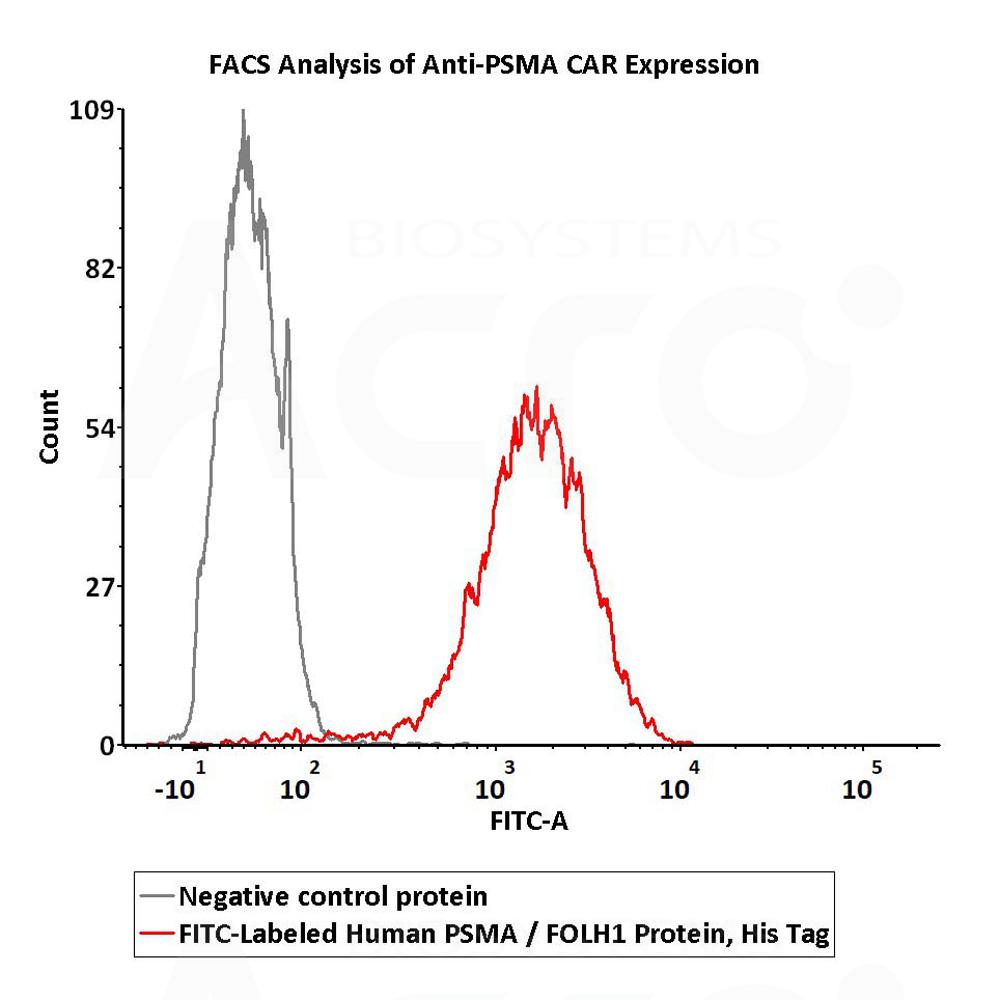
2e5 of Anti-PSMA CAR-293 cells were stained with 100 μL of 10 μg/mL of FITC-Labeled Human PSMA Protein, His Tag (Cat. No. PSA-HF244) and negative control protein respectively. FITC signal was used to evaluate the binding activity (QC tested).

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Capromab pendetide | lndium-CYT-356; Indium-111-CYT-356; CYT-356; 111In CYT-356 | Approved | Eusa Pharma | ProstaScint | United States | Prostatic Neoplasms | Cytogen Corp | 1996-10-28 | Prostatic Neoplasms | Details |
| Piflufolastat F 18 | Approved | Johns Hopkins University | Pylarify, Pylclari | United States | Prostatic Neoplasms | Progenics Pharmaceuticals Inc | 2021-05-26 | Carcinoma, Renal Cell; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Genital Neoplasms, Female; Diagnostic agents; Metastatic breast cancer; Castration-Sensitive Prostate Neoplasms; Carcinoma, Hepatocellular; Neoplasm Metastasis | Details | |
| Ga-68 PSMA-11 | Ga-68-PSMA-11; Ga-68-PSMA; 68Ga-HBED-CC-PSMA11; AAA517; [68Ga]Ga-PSMA-11; AAA-517 | Approved | Radiomedix Inc | Gallium Ga 68 Psma-11, Illuccix, ILLUCCIX, Locametz | United States | Prostatic Neoplasms | University Of California, San Francisco Foundation, Telix Pharmaceuticals Ltd | 2020-12-01 | Neoplasm Recurrence, Local; Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Diagnostic agents; Carcinoma, Hepatocellular; Neoplasm Metastasis | Details |
| Lutetium (177Lu) vipivotide tetraxetan | 177-Lu-PSMA-617; AAA-617; Lu-177- RLT; PSMA-617-[177Lu]; Lutetium-177-PSMA-617 | Approved | Radiomedix Inc | PLUVICTO | United States | Prostatic Neoplasms, Castration-Resistant | Novartis Pharmaceuticals Corp | 2022-03-23 | Carcinoma, Renal Cell; Carcinoma, Verrucous; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Adenoid Cystic; Prostatic Neoplasms; Carcinoma, Squamous Cell; Neoplasm Metastasis | Details |
| Flotufolastat F-18 | (18F)-rhPSMA-7.3; 18FrhPSMA-7.3; 18F-rhPSMA-7.3; F-18-rhPSMA-7.3; Fluorine-18 rhPSMA; rhPSMA-7.3 (18F) | Approved | Blue Earth Diagnostics Inc, Technical University Munich | POSLUMA | United States | Prostatic Neoplasms | Blue Earth Diagnostics Ltd | 2023-05-25 | Prostatic Neoplasms; Contrast agents | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| 225 Actinium PSMA-617 | 225 Actinium PSMA-617; 225Ac-PSMA 617; AAA 817; 225Ac-PSMA-617 | Phase 3 Clinical | Novartis Pharma Ag, Endocyte Inc, Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 18F-PSMA-1007 | 18F-PSMA-1007 | Phase 3 Clinical | Deutsches Krebsforschungszentrum | Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms | Details |
| Gallium-68 PSMA-617 | 225 Ac-PSMA-617; 68Ga-PSMA-617; 68Ga-DKFZ-617; 68Ga-DKFZ-PSMA -617; Gallium-68-PSMA-617; PSMA-617-[68Ga]; 68Ga-DOTA-PSMA-DKFZ -617 | Phase 3 Clinical | Peking Union Medical College Hospital | Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details |
| Vidoflufolastat (18F) | CTT-1057-F-18; CTT-1057-18F; 18F-CTT1057; CTT-1057 | Phase 3 Clinical | Washington University, Cancer Targeted Technology Llc | Carcinoma, Renal Cell; Prostatic Neoplasms | Details |
| Lutetium-177 zadavotide guraxetan | Phase 3 Clinical | Curium Us Llc | Prostatic Neoplasms | Details | |
| Nizaracianine triflutate | ZW800-1 | Phase 3 Clinical | Leiden University Medical Center, Curadel Surgical Innovations Inc | Ureteral Diseases | Details |
| ITM-24D | ITM-24D; 68Ga-PSMA-TTM | Phase 3 Clinical | ITM Isotope Technologies Munich SE | Prostatic Neoplasms | Details |
| 18F vipivotide tetraxetan | 18F-PSMA-617; Al18F-PSMA-617 | Phase 3 Clinical | Peking Union Medical College Hospital | Prostatic Neoplasms | Details |
| 64Cu-PSMA I&T | Copper Cu 64 PSMA I&T; Cu-64-PSMA-I&T; 64-Copper-PSMA-I&T; Copper-64-prostate specific membrane antigen I&T | Phase 3 Clinical | Curium Us Llc | Prostatic Neoplasms | Details |
| Thretide[18F] | 18F-LNC1001; 18F-LNC-1001 | Phase 3 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Neoplasms; Prostatic Neoplasms; Diagnostic agents | Details |
| 64Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 3 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-DOTA-rosopatamb | 177Lu-DOTA-TLX591-CHO; TLX591 | Phase 3 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [Lu177]-PNT-2002 | PNT-2002 | Phase 3 Clinical | Point Biopharma Inc | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details |
| Lutetium-177-PSMA-I&T | Phase 2 Clinical | Radboud University Nijmegen | Salivary Gland Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details | |
| 177Lu-PSMA-R2 | 177Lu-PSMA-R2; 177Lu-PSMA-SR6; AAA-602 | Phase 2 Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 89Zr-DFO-huJ591 | Zr89-J591; 89Zr-DFO-J591; 89Zr-DFO-huJ591 | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center | Prostatic Neoplasms | Details |
| Nezastomig | REGN-5678 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 225Ac-J591 | 225Ac-DOTA-J591; 225Ac-J591; CONV 01-α; J591-Ac-225 | Phase 2 Clinical | Cornell University | Prostatic Neoplasms | Details |
| ATL-101 | MLN-591RL; ATL-101; huJ-591; MLN-591; J-591; Lu-177-J591; 177Lu-J591; 90Y-J591; muJ-591 | Phase 2 Clinical | Weill Medical College of Cornell University | Ovarian Neoplasms; Head and Neck Neoplasms; Kidney Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| [131I]MIP-1095 | 1095; [131I]MIP-1095; [131I]MIP-1466; 131I-MIP-1095; 131-I-MIP-1466; 131I-MIP-1466 | Phase 2 Clinical | Progenics Pharmaceuticals Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| CONV-01-alpha + PSMA I&T | Phase 2 Clinical | Convergent Therapeutics Inc | Prostatic Neoplasms | Details | |
| LAVA-1207 | LAVA-1207 | Phase 2 Clinical | Vu University Medical Center, Lava Therapeutics NV | Prostatic Neoplasms, Castration-Resistant | Details |
| RHN001-Dx (99mTc-PSMA) | technetium-99m (99mTc); Technetium 99m-RHN 001; 99mTc-RHN001 | Phase 2 Clinical | Heidelberg University Hospital, Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| RHN001-Tx (188Re-PSMA) | RHN001-Tx (188Re-PSMA); rhenium-188- RHN 001; Rhenium-188 RHN 001 (188Re) | Phase 2 Clinical | Telix Pharmaceuticals Ltd, Heidelberg University Hospital | Prostatic Neoplasms | Details |
| In-111 rosopatamab tetraxetan | Phase 2 Clinical | Convergent Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant | Details | |
| Ac-225 rosopatamab tetraxetan | Phase 2 Clinical | Convergent Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant | Details | |
| DGPR-1008 | DGPR1008; DGPR-1008 | Phase 2 Clinical | Xindou Biotechnology (Suzhou) Co Ltd | Contrast agents; Prostatic Neoplasms | Details |
| INR-101 | INR101; INR-101; INR101 PET CT; INR101-PET/CT | Phase 2 Clinical | Cloud Nuclear Medicine (Tianjin) Co Ltd | Prostatic Neoplasms; Diagnostic agents | Details |
| 68Ga-NYM032 | 68Ga-NYM032; 68GaNYM032 | Phase 2 Clinical | Wuxi Nuoyu Pharmaceutical Technology Co Ltd | Prostatic Neoplasms; Contrast agents | Details |
| 177Lu-NYM032 | 177Lu-NYM032; 177LuNYM032 | Phase 2 Clinical | Wuxi Nuoyu Pharmaceutical Technology Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| AMX-500 | SAR-446329; SAR446329; VIR-5500; AMX-500 | Phase 2 Clinical | Amunix Pharmaceuticals Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| [225Ac]Ac-PSMA-R2 | AAA802; [225Ac]Ac-PSMA-R2; AAA-802 | Phase 2 Clinical | Novartis Pharma Ag | Prostatic Neoplasms | Details |
| 177Lu-PSMA | 177Lu-PSMA | Phase 2 Clinical | Istituto Scientifico Romagnolo Per Lo Studio E La Cura Dei Tumori | Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms; Neoplasm Metastasis | Details |
| [177Lu]JH020002 | Lu 177 JH020002; [177Lu]JH020002; 177-Lu JH-020002 | Phase 2 Clinical | Bivision Biomedical Technology (Nanjing) Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [177Lu]Lu-XT033 | [177Lu]Lu-XT033 | Phase 2 Clinical | Beijing Xiantong International Technology Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 177Lu-HTK03170 | 177Lu-HTK03170 | Phase 2 Clinical | British Columbia Cancer Agency | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| Ga-68-NGUL | Phase 2 Clinical | Cellbion Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| CD19/70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| GD2/PSMA Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR GD2/PSMA | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Solid tumours | Details |
| PSMA/CD70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR PSMA/CD70 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Neoplasms | Details |
| 177Lu-rhPSMA-10.1 | 177Lu-rhPSMA10.1; (177Lu) rhPSMA-10.1; Lutetium (177Lu) rhPSMA-10.1 (Tx IMP); Lutetium-177 rhPSMA10.1; Lutetium Lu 177 PSMA-10.1; 177Lu rhPSMA-10.1; Lu177-rhPSMA; 177Lu Radiohybrid PSMA-10.1; 177Lu-rhPSMA-10.1; 177Lu-rhPSMA | Phase 2 Clinical | Technical University Munich | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Urogenital Neoplasms; Prostatic Diseases | Details |
| Lu-177-DGUL | PSMA-D GUL; Lu-177-DGUL; 177Lu-DOTA-GUL | Phase 2 Clinical | Cellbion Co Ltd | Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 68Ga-PSMA-IRDye | TLX591-Sx | Phase 2 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| [177Lu]Ludotadipep | 177Lu-FC705; 177Lu-FC-705 | Phase 2 Clinical | Futurechem | Prostatic Neoplasms, Castration-Resistant | Details |
| 67Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 2 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant | Details | |
| IS-002 | IS-002 | Phase 2 Clinical | Intuitive Surgical Medical Device Science & Technology (Shanghai) Co Ltd | Prostatic Neoplasms | Details |
| OTL-78 | OTL-78 | Phase 2 Clinical | Purdue University, Novartis Pharma Ag | Prostatic Neoplasms | Details |
| INO-5401 | INO-5401 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Glioblastoma; Carcinoma, Transitional Cell | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| P28z-CAR | P28z-CAR | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center, United States Department Of Defense | Prostatic Neoplasms | Details |
| PSMA-CART cell therapy (Shanghai Bioray Laboratory) | Phase 1 Clinical | BRL Medicine Inc | Prostatic Neoplasms, Castration-Resistant | Details | |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| 177Lu-EB-vipivotide tetraxetan | 177-Lu-EB-PSMA-617 | Phase 1 Clinical | Peking Union Medical College Hospital, National Institute For Biomedical Imaging And Bioengineering (Nibib) | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details |
| [177Lu]-CTT-1403 | CTT-1403; [177Lu]CTT1403; 177Lutetium CTT1403; [177Lu]CTT-1403 | Phase 1 Clinical | Cancer Targeted Technology Llc | Prostatic Neoplasms | Details |
| Pelgifatamab corixetan | BAY-2315497 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| [18F]F-DCFPyL | [18F]F-DCFPyL | Phase 1 Clinical | Anhui Provincial Hospital | Prostatic Neoplasms | Details |
| [18F]AlF-PSMA-N5 | [18F]AlF-PSMA-N5 | Phase 1 Clinical | Anhui Provincial Hospital | Prostatic Neoplasms | Details |
| AS-1986NS | Phase 1 Clinical | Antelope Surgical Solutions Inc | Prostatic Neoplasms | Details | |
| [177Lu]Lu-BQ7876 | Lutetium-177 Labeled BQ7876; [177Lu]Lu-BQ7876 | Phase 1 Clinical | Tomsk National Research Medical Center Of The Russian Academy Of Sciences | Prostatic Neoplasms | Details |
| 68Ga-AAZTA-NI-093 | Phase 1 Clinical | First Affiliated Hospital Of Fujian Medical University | Prostatic Neoplasms | Details | |
| [177Lu]Lu-PSMA-137 | [177Lu]Lu-PSMA-137 | Phase 1 Clinical | The First Affiliated Hospital Of Beijing University | Prostatic Neoplasms | Details |
| 68Ga-PSMA-0057 | Phase 1 Clinical | Suzhou Ruihe Pharmaceutical Technology Co Ltd | Prostatic Neoplasms | Details | |
| IR800-IAB2MA | IR800-IAB2MA | Phase 1 Clinical | Imaginab Inc | Kidney Neoplasms; Prostatic Neoplasms | Details |
| [18F]F-PSMA-N5 | [18F]F-PSMA-N5 | Phase 1 Clinical | Anhui Provincial Hospital | Prostatic Neoplasms | Details |
| PSW-1025 | PSW-1025 | Phase 1 Clinical | Osaka University, Japan Agency For Medical Research And Development | Prostatic Neoplasms | Details |
| 68Ga-AAZTA-093 | 68Ga-AAZTA-093 | Phase 1 Clinical | First Affiliated Hospital Of Fujian Medical University | Prostatic Neoplasms | Details |
| 99mTc-QULIC-5-P1 | 99mTc-QULIC-5-P1 | Phase 1 Clinical | First Affiliated Hospital Of Chongqing Medical University | Prostatic Neoplasms | Details |
| 68Ga-PSFA | 68Ga-PSFA | Phase 1 Clinical | First Affiliated Hospital Of Chongqing Medical University | Adenomatous Polyposis Coli | Details |
| 161Tb-SibuDAB | Phase 1 Clinical | University Hospital Basel | Prostatic Neoplasms, Castration-Resistant | Details | |
| Actinium-225 FL 020 (Full-Life Technologies) | 225-Ac FL-020; 225Ac-FL-020 | Phase 1 Clinical | Full Life Technologies Ltd | Prostatic Neoplasms, Castration-Resistant | Details |
| [Ac-225]-PSMA-62 | [Ac-225]-PSMA-62 | Phase 1 Clinical | Point Biopharma Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Hormone-Sensitive Prostate Neoplasms | Details |
| 111In-PSMA-Trillium | BAY-3632687; BAY3632687 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant; Prostatism | Details |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| ABBV-969 | ABBV-969; ABBV969 | Phase 1 Clinical | Abbvie Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| BAY-3563254 | BAY-3563254; BAY3563254 | Phase 1 Clinical | PSMA Therapeutics Inc, Noria Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatism; Prostatic Neoplasms | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| Actinium-225-macropa-pelgifatamab | BAY-3546828; BAY3546828 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-PSMA-0057 | 177Lu-PSMA-0057 | Phase 1 Clinical | Nanjing First Hospital, Nanjing Medical University | Prostatic Neoplasms | Details |
| [68Ga]Ga-PSMA-D5 | [68Ga]Ga-PSMA-D5 | Phase 1 Clinical | Anhui Provincial Hospital | Prostatic Neoplasms | Details |
| 177Lu-LNC1003 | 177Lu-LNC1003 | Phase 1 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| PSMA-Targeted [In-111]-Labeled Trillium Compound | Phase 1 Clinical | Ratio Therapeutics Inc | Prostatic Neoplasms | Details | |
| Autologous T cells therapy(Unicar-Therapy) | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Prostatic Neoplasms | Details | |
| 212Pb-NG001 | 212Pb-NG001; AB-001; AB001 | Phase 1 Clinical | ARTBIO Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CART-PSMA cells(Nova Therapeutics) | Phase 1 Clinical | Nova Therapeutics LLC | Prostatic Neoplasms | Details | |
| 68Ga-NY108 | 68-Ga-labeled NY-108; 68-Ga-labeled NYM032; 68Ga-NYM032 | Phase 1 Clinical | Affiliated Hospital of Jiangnan University | Prostatic Neoplasms | Details |
| 177Lu-P17-088 | 177Lu-P17-088 | Phase 1 Clinical | Peking Union Medical College Hospital | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-P17-087 | 177Lu-P17-087 | Phase 1 Clinical | Peking Union Medical College Hospital | Prostatic Neoplasms, Castration-Resistant | Details |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| JNJ-80038114 | JNJ-80038114 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| CART-PSMA-TGFbRDN (University of Pennsylvania) | Phase 1 Clinical | University Of Pennsylvania | Prostatic Neoplasms | Details | |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CB-307 | CB-307; CB307 | Phase 1 Clinical | Crescendo Bioscience Inc | Neoplasms; Prostatic Neoplasms, Castration-Resistant | Details |
| Copper 64-DOTA-TLX 592 | 64Cu-TLX-592; 64Cu-DOTA-TLX592 | Phase 1 Clinical | Telix International Pty Ltd | Prostatic Neoplasms | Details |
| ARX-517 | ARX-517; ARX517 | Phase 1 Clinical | Ambrx Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| AVC-102 | AVC-102 | Phase 1 Clinical | AvenCell Therapeutics Inc | Kidney Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CBP-1018 | CBP-1018 | Phase 1 Clinical | Coherent Biopharma Suzhou Co Ltd | Solid tumours; Carcinoma, Renal Cell; Prostatic Neoplasms; Lung Neoplasms | Details |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Anti-PSMA CAR T-cell therapy (TNK Therapeutics) | Phase 1 Clinical | Sorrento Therapeutics Inc | Neoplasms | Details | |
| CART-PSMA-TGFβRDN cell therapy (Tmunity Therapeutics) | TmPSMA-02 | Phase 1 Clinical | Tmunity Therapeutics Inc, University Of Minnesota, University Of Pennsylvania | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 68Ga-PSMA-R2 (Advanced Accelerator Applications SA) | Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms | Details | |
| [At-211] PSMA-5 | [At-211] PSMA-5 | Clinical | Osaka University | Prostatic Neoplasms | Details |
This web search service is supported by Google Inc.







