
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































Mouse HGF R, Fc Tag is fused with Fc fragment of human IgG1 at the C-terminus. The mature form of HGF R is a disulfide-linked heterodimer composed of proteolytically cleaved α and β chain. Each α and β chain has a calculated MW of 32.1 kDa (α chain) and 95.1 kDa (β chain Fc chimera). The protein migrates as 40-45 kDa (α chain) and 125-135 kDa (β chain Fc chimera) under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
Lyophilized from 0.22 μm filtered solution in 50 mM Tris, 100 mM Glycine, 25 mM Arginine, 150 mM NaCl, pH7.5 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
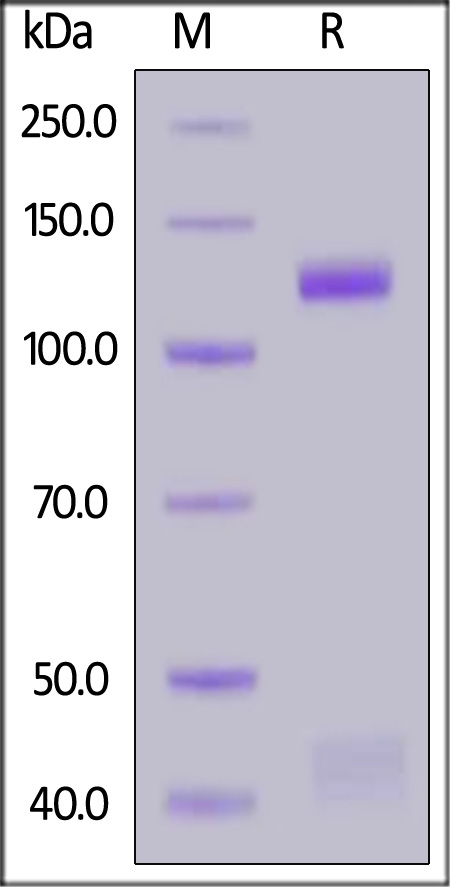
Mouse HGF R, Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.

The purity of Mouse HGF R, Fc Tag (Cat. No. MET-M5254) is more than 90% and the molecular weight of this protein is around 254-311 kDa verified by SEC-MALS.
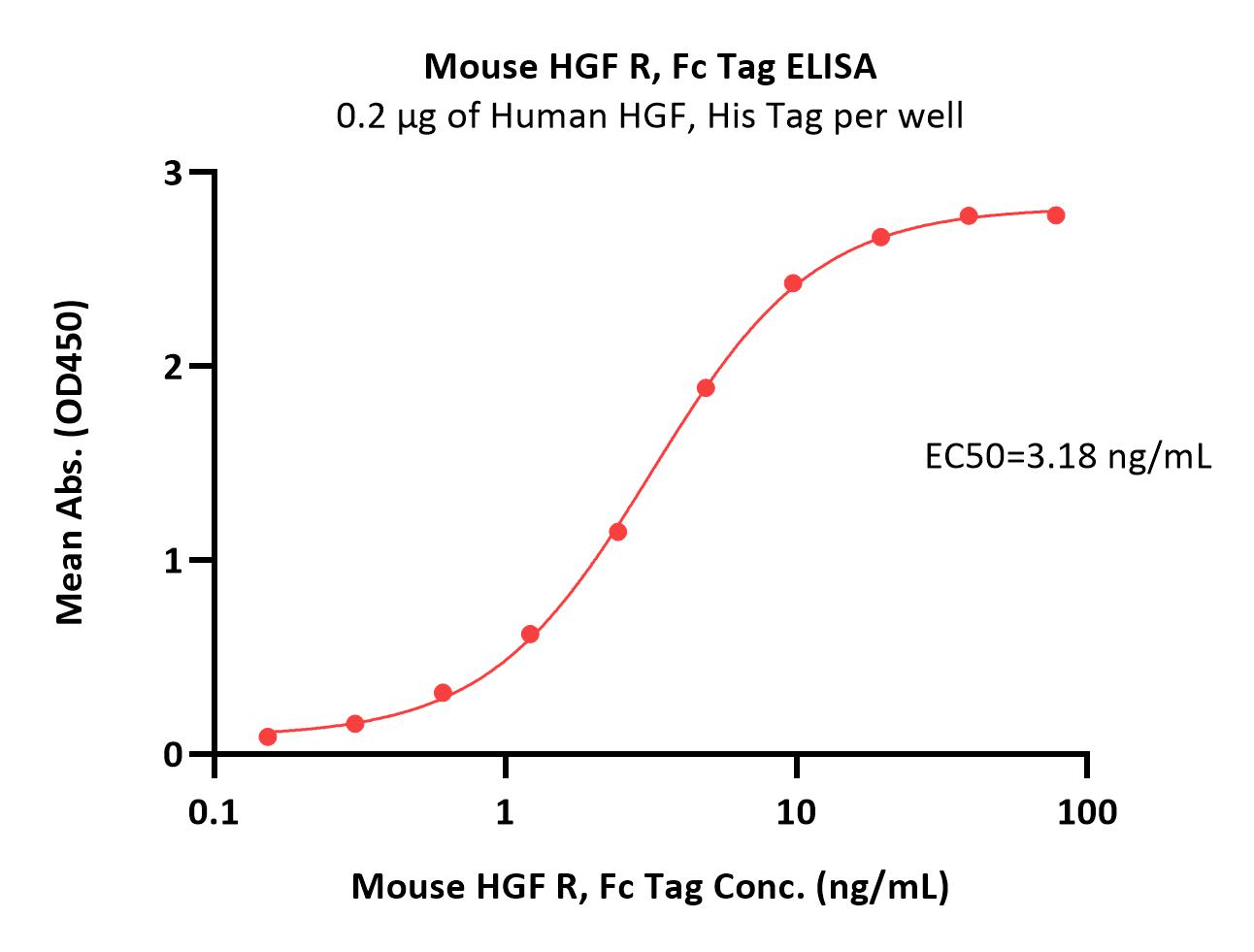
Immobilized Human HGF, His Tag (Cat. No. HGF-H52H3) at 2 μg/mL (100 μL/well) can bind Mouse HGF R, Fc Tag (Cat. No. MET-M5254) with a linear range of 0.2-10 ng/mL (QC tested).

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Envonalkib | TQ-B3139; CT-1139; CT-711 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd, Beijing Centaurus Biopharma Co Ltd | 安洛晴 | Mainland China | Carcinoma, Non-Small-Cell Lung | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2024-06-11 | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Crizotinib | PF-1066; PF-2341066; PF-02341066; PF-002341066 | Approved | Pfizer Inc | 赛可瑞, Xalkori | United States | Carcinoma, Non-Small-Cell Lung | Pf Prism Cv | 2011-08-26 | Urethral Neoplasms; Neoplasm Metastasis; Adenocarcinoma; Neurilemmoma; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Granuloma, Plasma Cell; Endometrial Neoplasms; Lymphoma, Large-Cell, Anaplastic; Lymphoma; Ureteral Neoplasms; Solid tumours; Lymphoma, Primary Cutaneous Anaplastic Large Cell; Neuroblastoma; Urinary Bladder Neoplasms; Myofibroma; Uveal melanoma; Multiple Myeloma; Neurofibromatosis 2; Neoplasms; Renal Insufficiency; Hematologic Neoplasms | Details |
| Unecritinib | TQ-B3101; TTQ-B3101 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd, Southeast University | 安柏尼 | Mainland China | Carcinoma, Non-Small-Cell Lung | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2024-04-24 | Stomach Neoplasms; Neoplasms; Lymphoma, Large-Cell, Anaplastic; Lymphoma; Carcinoma, Non-Small-Cell Lung | Details |
| Glumetinib | HH-SCC244; SCC244; SCC-244 | Approved | Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences, ShangHai HaiHe Biopharma Co Ltd | 海益坦, Haiyitan | Mainland China | Carcinoma, Non-Small-Cell Lung | ShangHai HaiHe Biopharma Co Ltd | 2023-03-07 | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| Tepotinib hydrochloride hydrate | MSC-2156119J; MSC-2156119; EMD-1214063 | Approved | Merck Serono | Tepmetko, テプミトコ, TEPMETKO | Japan | Carcinoma, Non-Small-Cell Lung | Merck KGaA, Darmstadt, Germany | 2020-03-25 | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Capmatinib Hydrochloride | INCB-28060; C2A374O70X; INC-280; INCB-028060; NVP-INC280-NX | Approved | Incyte Corp, Novartis Pharma Ag | Tabrecta | United States | Carcinoma, Non-Small-Cell Lung | Novartis Pharmaceuticals Corp | 2020-05-06 | Adenocarcinoma of Lung; Neoplasm Metastasis; Carcinoma, Hepatocellular; Melanoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Gliosarcoma; Colorectal Neoplasms; Sarcoma; Solid tumours; Neoplasms; Triple Negative Breast Neoplasms; Glioblastoma; Carcinoma; Carcinoma, Renal Cell; Kidney Neoplasms; Liver Neoplasms | Details |
| Savolitinib | HM-5016504; AZD-6094; HMP-504; HMPL-504 | Approved | Hutchison Medipharma Ltd | 沃瑞沙, ORPATHYS | Mainland China | Carcinoma, Non-Small-Cell Lung | Hutchison Medipharma Ltd | 2021-06-22 | Central Nervous System Neoplasms; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Urologic Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Diffuse Intrinsic Pontine Glioma; Cholangiocarcinoma; Medulloblastoma; Kidney Diseases; Neoplasms; Colonic Neoplasms; Carcinoma; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Kidney Neoplasms; Solid tumours | Details |
| Ensartinib Hydrochloride | X-396 | Approved | Xcovery | 贝美纳, ENSACOVE | Mainland China | Carcinoma, Non-Small-Cell Lung | Betta Pharmaceuticals Co Ltd | 2020-11-17 | Neuroblastoma; Xanthogranuloma, Juvenile; Neuroectodermal Tumors, Primitive, Peripheral; Neoplasms, Germ Cell and Embryonal; Carcinoma, Non-Small-Cell Lung; Carcinoma, Squamous Cell; Lymphoma, Non-Hodgkin; Lung Neoplasms; Glioma; Brain metastases; Histiocytic Sarcoma; Sarcoma, Ewing; Solid tumours; Osteosarcoma; Histiocytosis, Langerhans-Cell; Sarcoma; Central Nervous System Neoplasms; Neoplasms; Wilms Tumor; Rhabdoid Tumor; Hepatoblastoma; Medulloblastoma; Ependymoma; Rhabdomyosarcoma | Details |
| Amivantamab | JNJ-61186372; JNJ-372; JNJ-6372; CNTO4424; CNTO-4424 | Approved | Genmab A/S, Johnson & Johnson Innovative Medicine | RYBREVANT | United States | Carcinoma, Non-Small-Cell Lung | Janssen Biotech Inc | 2021-05-21 | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Glioblastoma; Carcinoma, Adenoid Cystic; Colorectal Neoplasms; Lung Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Hepatocellular | Details |
| Cabozantinib S-malate | XL-184; BMS-907351; RO7047650; RO-7047650 | Approved | Exelixis Inc | Cometriq, Cabometyx | United States | Thyroid Neoplasms; Carcinoma, Neuroendocrine | Exelixis Inc | 2012-11-29 | Neuroblastoma; Thyroid Neoplasms; Hepatic Insufficiency; Astrocytoma; Peritoneal Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Gliosarcoma; Urethral Neoplasms; Sarcoma, Clear Cell; Uterine Neoplasms; Cholangiocarcinoma; Neurofibroma, Plexiform; Brain Neoplasms; Adenocarcinoma, Clear Cell; Carcinoma, Adenosquamous; Medullary thyroid cancer (MTC); Sarcoma, Ewing; Sarcoma; Osteosarcoma; Sarcoma, Alveolar Soft Part; Meningioma; Melanoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Paraganglioma; Neoplasms, Germ Cell and Embryonal; Carcinoma, Hepatocellular; Prostatic Neoplasms; Glioma; Brain metastases; Endometrial Neoplasms; Leukemia, Myeloid, Acute; Carcinoma, Squamous Cell; Lymphoma; Carcinoma, Neuroendocrine; Fallopian Tube Neoplasms; Carcinoma, Renal Cell; Adenoma, Islet Cell; Glioblastoma; Carcinoma, Ovarian Epithelial; Renal Insufficiency; Hepatoblastoma; Pheochromocytoma; Carcinoid Tumor; Carcinoma, Merkel Cell; Rejection of liver transp | Details |
| Vebreltinib | PLB-1001; CBT-101; APL-101; CBI-3103 | Approved | Mainland China | Carcinoma, Non-Small-Cell Lung | Beijing Purunao Biotechnology Co Ltd | 2023-11-16 | Colonic Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Glioma; Thyroid Neoplasms; Lung Neoplasms; Hepatic Insufficiency; Astrocytoma; Brain Neoplasms; Solid tumours; Glioblastoma; Pancreatic Neoplasms; Ganglioglioma; Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Kidney Neoplasms | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| HS-10241 | HS-10241 | Phase 3 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours; Carcinoma; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Zanzalintinib | XL-092 | Phase 3 Clinical | Exelixis Inc | Solid tumours; Leiomyosarcoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Carcinoma, Transitional Cell; Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Colorectal Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Fosgonimeton | NDX-1017; ATH-1017; ATH 1017 | Phase 3 Clinical | M3 Biotechnology | Alzheimer Disease; Dementia; Parkinson Disease; Lewy Body Disease | Details |
| Sitravatinib | MGCD-516; IND-155305; MGCD-0516; MG-91516; MG-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Carcinoma, Hepatocellular; Endometrial Neoplasms; Lung Neoplasms; Metastatic breast cancer; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Carcinoma, Squamous Cell; Hepatic Insufficiency; Ureteral Neoplasms; Solid tumours; Lung Diseases; Liposarcoma; Uveal melanoma; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Kidney Neoplasms; Biliary Tract Neoplasms | Details |
| HS-20117 | HS-20117; PM1080; PM-1080 | Phase 3 Clinical | Solid tumours; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Telisotuzumab adizutecan | ABBV-400 | Phase 3 Clinical | Abbvie Inc | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Esophageal adenocarcinoma; Carcinoma, Pancreatic Ductal; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| SC-0011 | SC-0011 | Phase 3 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| AL-2846 | AL-2846 | Phase 3 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd, Advenchen Laboratories Nanjing Ltd | Neurofibromatoses; Neurilemmoma; Neurofibromatosis 1; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Thyroid Neoplasms; Colorectal Neoplasms; Prostatic Neoplasms; Medullary thyroid cancer (MTC); Solid tumours; Neurofibroma; Ganglioglioma; Pancreatic Neoplasms; Neoplasms; Stomach Neoplasms; Ovarian Neoplasms; Liver Neoplasms; Bone metastases | Details |
| Ningetinib Tosylate | CT-053-PTSA; CT-053 | Phase 2 Clinical | Hec Pharm Co Ltd | Solid tumours; Intestinal Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Pamvatamig | MCLA-129 | Phase 2 Clinical | Merus Nv | Solid tumours; Head and Neck Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Esophageal Squamous Cell Carcinoma; Carcinoma, Non-Small-Cell Lung | Details |
| Merestinib | 5OGS5K699E; LY-2801653 | Phase 2 Clinical | Eli Lilly And Company | Biliary Tract Neoplasms; Solid tumours; Skin Melanoma; Bone metastases; Pancreatic Neoplasms; Neoplasms; Microsatellite instability-high cancer; Breast Neoplasms; Cholangiocarcinoma; Lymphoma, Mantle-Cell; Colorectal Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Gallbladder Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Sym-015 | Sym-015 | Phase 2 Clinical | Symphogen A/S | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Vabametkib | KDDF-201603-08; ABN-401 | Phase 2 Clinical | Abion Inc | Solid tumours; Neoplasms | Details |
| REGN-5093-M114 | METxMET-M114; REGN5093-M114 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Carcinoma, Non-Small-Cell Lung | Details |
| Bafisontamab | FIT-013a; EMB-01 | Phase 2 Clinical | Shanghai Epimab Biotherapeutics, Inc | Biliary Tract Neoplasms; Liver Neoplasms; Stomach Neoplasms; Neoplasms; Digestive System Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Gastrointestinal Neoplasms | Details |
| GB-263 | GB-263; GB-263T | Phase 2 Clinical | Genor Biopharma Co Ltd | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Elzovantinib | TPX-0022 | Phase 2 Clinical | Turning Point Therapeutics Inc | Solid tumours; Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| PRO-1286 | PRO-1286; GEN1286; GEN-1286 | Phase 2 Clinical | ProfoundBio (Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| Do-1 | Do-1 | Phase 2 Clinical | DeuterOncology NV | Lung Neoplasms | Details |
| PFL-002 | VERT-002; PFL-002; PFL-002/VERT-002 | Phase 2 Clinical | Vertical Bio AG | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| EMI-137 | EMI-137; GE-137 | Phase 2 Clinical | Ge Healthcare | Esophageal Neoplasms; Extranodal Extension; Colonic Neoplasms; Barrett Esophagus | Details |
| Davutamig | REGN-5093 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Carcinoma, Non-Small-Cell Lung | Details |
| CKD-702 | Phase 2 Clinical | Chong Kun Dang Pharmaceutical Corp | Stomach Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant adenovirus-hepatocyte growth factor(Institute Of Radiation And Radiation Medicine, Chinese Academy Of Military Medical Sciences) | Phase 2 Clinical | Institute Of Radiation And Radiation Medicine, Chinese Academy Of Military Medical Sciences, Beijing Haitai United Pharmaceutical Technology Development Co Ltd | Myocardial Ischemia | Details | |
| RC-108 | RC-108; RC108 | Phase 2 Clinical | RemeGen Co Ltd | Solid tumours; Digestive System Neoplasms; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Emibetuzumab | LA-480; LY-2875358 | Phase 2 Clinical | Eli Lilly And Company | Solid tumours; Carcinoma, Renal Cell; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Lymphoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| SHR-A1403 | SHR-A1403 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Solid tumours | Details |
| E-3112 | E-3112; E-3112/CP1 | Phase 1 Clinical | Ea Pharma | Liver Diseases | Details |
| CM-118 | CM-118 | Phase 1 Clinical | Xcovery | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Kanitinib | CX-1003 | Phase 1 Clinical | Beijing Konruns Pharmaceutical Co Ltd, Guangzhou Yingsheng Bio Technology Co Ltd | Solid tumours | Details |
| BPI-9016 | BPI-9016; BPI-9016M | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| HLX-55 | HLX-55; KTN-0216 | Phase 1 Clinical | Kolltan Pharmaceuticals Inc | Solid tumours | Details |
| Pamufetinib | TAS-115 | Phase 1 Clinical | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | Neoplasms | Details |
| NP-01 (SSY Group) | Phase 1 Clinical | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Prostatic Neoplasms; Lung Neoplasms | Details | ||
| Ad-NK4 gene therapy (Chiba Cancer Center Res Inst/Toho University/Tokyo Women’s Medical University Yachiyo Medical Center) | Phase 1 Clinical | Mesothelioma | Details | ||
| Boxitinib Hydrochloride | Phase 1 Clinical | Hec Pharm Co Ltd | Carcinoma, Renal Cell; Stomach Neoplasms; Breast Neoplasms; Glioma; Lung Neoplasms | Details | |
| BYON-3521 | BYON-3521 | Phase 1 Clinical | Byondis Bv | Solid tumours; Neoplasms | Details |
| ATH-1020 | ATH-1020 | Phase 1 Clinical | Athira Pharma Inc | Details | |
| QBH-196 | QBH-196 | Phase 1 Clinical | Shenyang Pharmaceutical University | Solid tumours; Stomach Neoplasms | Details |
| BG-T187 | BG-T187; BGT187 | Phase 1 Clinical | BeOne Medicines Ltd | Solid tumours; Lung Neoplasms; Gastrointestinal Neoplasms | Details |
| KY-0301 | KY-0301 | Phase 1 Clinical | Novatim Immune Therapeutics (Zhejiang) Co Ltd | Solid tumours | Details |
| [11C]savolitinib | [11C]savolitinib | Phase 1 Clinical | Astrazeneca Plc | Details | |
| [14C]HS-10241 | [14C]HS-10241 | Phase 1 Clinical | Jiangsu Hansoh Pharmaceutical Group Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| YL-211 | YL211; YL-211; RG-6648; RG6648 | Phase 1 Clinical | MediLink Therapeutics Co Ltd | Solid tumours | Details |
| ABBV-303 | ABBV-303; DF-4101 | Phase 1 Clinical | Dragonfly Therapeutics Llc | Solid tumours | Details |
| FPI-2107 | [111In]-FPI-2107 | Phase 1 Clinical | Fusion Pharmaceuticals Inc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| FPI-2053 | FPI-2053 | Phase 1 Clinical | Fusion Pharmaceuticals Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| DM-005 | DM005; YH013; DM-005 | Phase 1 Clinical | Doma Biopharmaceutical (Suzhou) Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Non-Small-Cell Lung | Details |
| ANS-014004 | ANS014004; ANS-014004; ANS-01; ANS01 | Phase 1 Clinical | Solid tumours; Neoplasms | Details | |
| FPI-2068 | [225Ac]-FPI-2068; FPI-2068 | Phase 1 Clinical | Fusion Pharmaceuticals Inc, Astrazeneca Plc | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Colorectal Neoplasms; Carcinoma, Pancreatic Ductal; Carcinoma, Non-Small-Cell Lung | Details |
| SPH-3348 | SPH-3348; SPH3348; I-020 | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Solid tumours; Neoplasms | Details |
| MYTX-011 | MYTX-011 | Phase 1 Clinical | Mythic Therapeutics Inc | Carcinoma, Non-Small-Cell Lung | Details |
| AZD9592 | AZD-9592; AZD9592 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours; Head and Neck Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| ATH-1105 | ATH-1105 | Phase 1 Clinical | Athira Pharma Inc | Amyotrophic Lateral Sclerosis; Frontotemporal Dementia | Details |
| TAVO-412 | TAVO-412 | Phase 1 Clinical | Tavotek Biotherapeutics (Hong Kong) Ltd, Tuochuang Biotechnology (Jiangsu) Co Ltd | Solid tumours; Neoplasms | Details |
| NP-107 | NP107; NP-107 | Phase 1 Clinical | Shanghai Nawei Biotechnology Co Ltd | Solid tumours | Details |
| ASKC-202 | ASKC202 | Phase 1 Clinical | Jiangsu Aosaikang Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| LMV-12(HE003) | LMV-12(HE003) | Phase 1 Clinical | Nanchang Hongyi Technology Co Ltd | Solid tumours | Details |
| GST-HG161 | GST-HG161 | Phase 1 Clinical | Wuxi Apptec Co Ltd, Fujian Cosunter Pharmaceutical Co Ltd | Solid tumours; Neoplasms; Carcinoma, Hepatocellular | Details |
| Metatinib Trometamol | BMS-817378; SIM-817378 | Phase 1 Clinical | Bristol-Myers Squibb Company | Solid tumours; Liver Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| NP-01 (Shijiazhuang No.4 Pharmaceutical/Nanjing Nadingfei Medical Technology) | NP-01 | Phase 1 Clinical | Shijiazhuang No 4 Pharmaceutical Co Ltd, Nanjing Nadingfei Pharmaceutical Technology Co Ltd | Liver Neoplasms; Solid tumours; Stomach Neoplasms; Lung Neoplasms | Details |
| SAIT-301 | SAIT-301 | Phase 1 Clinical | Samsung Medical Center | Neoplasms | Details |
| NPS-1034 | HOPE-777; KDDF-201111-02 | Neopharma Ltd | Details |
This web search service is supported by Google Inc.







