
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































Virus-like particles(VLPs) are formed by self-assembly of envelop/capsid proteins from viruses. Membrane Proteins can be constituted in-situ with VLPs produced from HEK293 cell cultures. These VLPs concentrate conformationally intact membrane proteins directly on the cell surface and produce soluble, high-concentration proteins perfect for immunization and antibody screening.

The VLPs provide the display of properly folded membrane proteins in their native cellular membrane in a compact size of 100~300 nm diameter (similar to the size of most viruses) making it optimal targets for dendritic cells in vivo and surface attachment for phage display.
The VLPs are highly immunogenic, so the immunization strategy should be optimized (antigen dose, regimen and adjuvant).
Supplied as 0.2 μm filtered solution in PBS, Arginine, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
This product is supplied and shipped with dry ice, please inquire the shipping cost.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
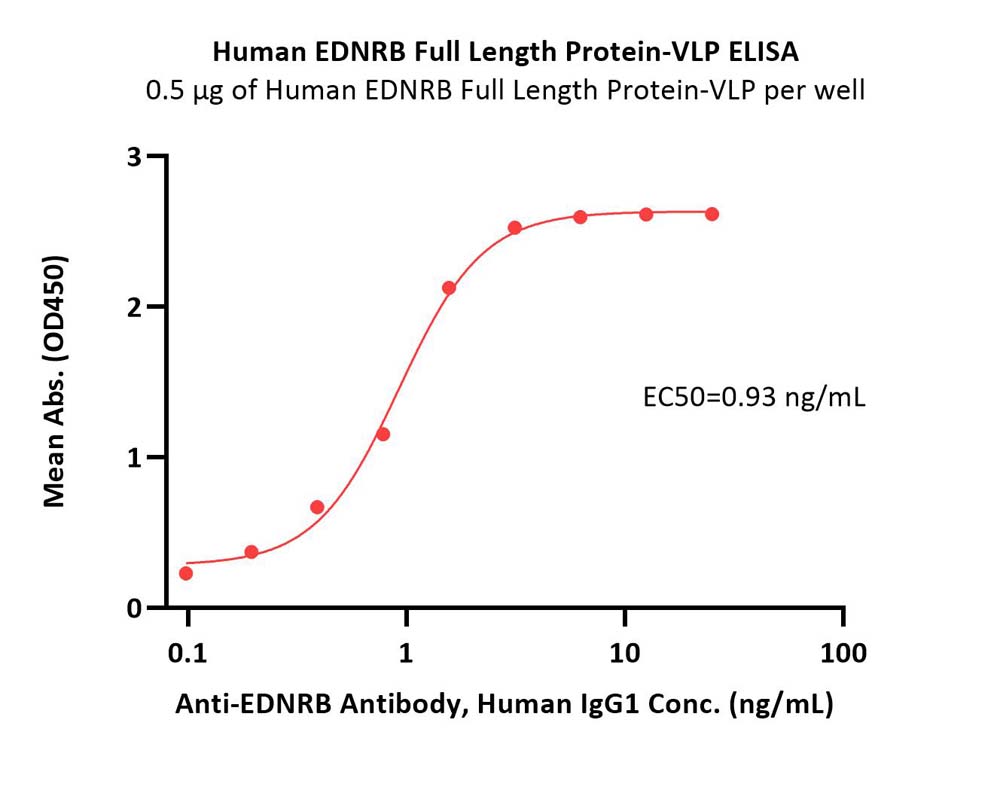
Immobilized Human EDNRB Full Length Protein-VLP (Cat. No. EDB-H52P7) at 5 μg/mL (100 μL/well) can bind Anti-EDNRB Antibody, Human IgG1 with a linear range of 0.1-2 ng/mL (QC tested).

The mean peak Radius of VLP is 65-85 nm with more than 95% intensity as determined by dynamic light scattering (DLS).
Price(USD) : $620.00
Price(USD) : $1235.00
Price(USD) : $4305.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Sovateltide | IRL-1620; PMZ-1620; SPI-1620 | Approved | University Of Illinois At Chicago | Tyvalzi | India | Stroke | Pharmazz Inc | 2023-05-31 | Biliary Tract Neoplasms; Brain Ischemia; Carcinoma; Spinal Cord Injuries; Asphyxia Neonatorum; Brain Diseases; Stroke; Ischemic Stroke; Dementia; Alzheimer Disease; Hypoxia-Ischemia, Brain; Cerebral Infarction; Carcinoma, Non-Small-Cell Lung | Details |
| Macitentan/Tadalafil | Approved | Actelion Pharmaceuticals Ltd | OPSYNVI, Yuvanci | United States | Pulmonary Arterial Hypertension | Actelion Pharmaceuticals Us Inc | 2024-03-22 | Phenylketonurias; Pulmonary Arterial Hypertension | Details | |
| Bosentan Hydrate | ACT-050088; HGP-1206; Ro-47-0203; Ro-47-0203/029; Ro-47-0203/039 | Approved | F. Hoffmann-La Roche Ltd | Stayveer, 全可利, Tracleer | United States | Pulmonary Arterial Hypertension | Actelion Pharmaceuticals Ltd | 2001-11-20 | Hepatopulmonary Syndrome; Diabetes Mellitus; Heart Defects, Congenital; Anemia, Sickle Cell; Persistent Fetal Circulation Syndrome; Ulcer; Glaucoma; Sleep Apnea Syndromes; Pulmonary Arterial Hypertension; Coronary Vasospasm; Lung Diseases, Interstitial; Hypertension, Pulmonary; Pulmonary Fibrosis; Hypertension; Fibrosis; Chronic thromboembolic pulmonary hypertension; Liver Cirrhosis; Sarcoidosis; Scleroderma, Systemic; Idiopathic Pulmonary Fibrosis; Diabetes Mellitus, Type 2; Heart Septal Defects, Atrial | Details |
| Macitentan | ACT-064992; Actelion-1; JNJ-67896062 | Approved | Actelion Pharmaceuticals Ltd | Zependo, Opsumit, 傲朴舒 | United States | Pulmonary Arterial Hypertension | Actelion Pharmaceuticals Us Inc | 2013-10-18 | Hypertension, Pulmonary; Pulmonary Embolism; Heart Failure, Diastolic; Heart Failure; Scleroderma, Systemic; Chronic thromboembolic pulmonary hypertension; Idiopathic Pulmonary Fibrosis; Glioblastoma; Thromboembolism; Pulmonary Arterial Hypertension; Eisenmenger Complex; Scleroderma-associated interstitial lung diseases; Ulcer; Heart Defects, Congenital; Anemia, Sickle Cell; Chronic Disease | Details |
| Aprocitentan | JNJ-2820; ACT-132577; AC-080 | Approved | Actelion Pharmaceuticals Ltd, Idorsia Pharmaceuticals Ltd | TRYVIO, JERAYGO | United States | Hypertension | Idorsia Pharmaceuticals Ltd | 2024-03-19 | Hypertension; Essential Hypertension; Hepatic Insufficiency; Renal Insufficiency, Chronic | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| VRP-1620 | VRP-1620 | Phase 3 Clinical | University Of Illinois | Breast Neoplasms | Details |
| Vodudeutentan | ENB-003; ENB-006 | Phase 2 Clinical | Enb Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Pancreatic Neoplasms; Neoplasms; Melanoma | Details |
| BQ-788 | BQ-788 | Phase 1 Clinical | Merck Serono | Hypertension; Cardiovascular Diseases; Melanoma | Details |
This web search service is supported by Google Inc.







