
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Assay Type | Sandwich-ELISA |
| Analyte | DLL4 |
| Format | 96T(8×12 strips) |
| Reactivity | Human |
| Regulatory Status | RUO |
| Sensitivity | < 18.75 pg/mL |
| Standard Curve Range | 18.75 pg/mL-1200 pg/mL |
| Assay Time | 3 hr 20 min |
| Suitable Sample Type | For the quantitative determination of human DLL4 in Cell Culture Supernatants, Plasma, Serum. |
| Sample volume | 100 uL |
Find the expiration date on the outside packaging and do not use reagents past their expiration date.
The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
| ID | Components | Size |
| RES052-C01 | Pre-coated Anti-DLL4 Antibody Microplate | 1 plate(8×12 strips) |
| RES052-C02 | Human Fc Tag DLL4 Standard | 20 μg |
| RES052-C03 | Biotin-Anti-DLL4 Antibody | 150 μL |
| RES052-C04 | Streptavidin-HRP | 50 μL |
| RES052-C05 | 10xWashing Buffer | 50 mL |
| RES052-C06 | 2xDilution Buffer | 50 mL |
| RES052-C07 | Substrate Solution | 12 mL |
| RES052-C08 | Stop Solution | 7 mL |

For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
To assess the linearity of the assay, samples spiked with high concentrations of human Fc Tag DLL4 were serially diluted with calibrator diluent to produce samples with values within the dynamic range of the assay.
Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision, Intra-Assay Precision CV<10%.

Three samples of known concentration were tested in three separate assays to assess inter-assay precision, Inter-Assay Precision CV<10%.

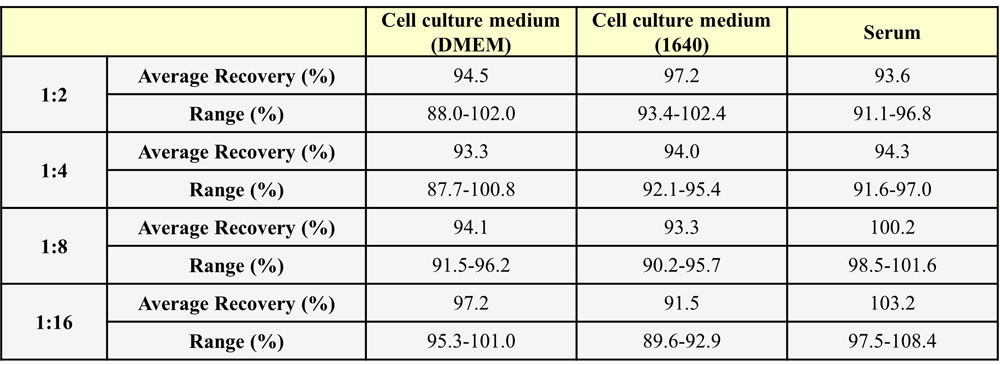
Three parts of blank serum were added with different concentrations of human Fc Tag DLL4, and the serum without human Fc Tag DLL4 was used as background to calculate the recovery rate. The range of the recovery rate is 83.7-116.5%, and the average recovery is 98.6%.


Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| TR-009 | NOV-1501; ABL001-ABL Bio; CTX-009; HD-B001A; ABL-001-ABL Bio; HDB001A; TR-009; ES-104 | Phase 3 Clinical | Abl Bio Inc | Biliary Tract Neoplasms; Solid tumours; Rectal Neoplasms; Colonic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Bile Duct Neoplasms; Ampullary Carcinoma; Gallbladder Neoplasms | Details |
| Navicixizumab | OMP-305B83 | Phase 3 Clinical | Oncomed Pharmaceuticals Inc | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Triple Negative Breast Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Fallopian Tube Neoplasms | Details |
| Dilpacimab | ABT-165; DVD-Ig ABT-165 | Phase 1 Clinical | Abbvie Inc | Solid tumours; Neoplasms | Details |
This web search service is supported by Google Inc.







