
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 78.2 kDa. The protein migrates as 55 kDa,65 kDa and 85-95 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
>90% as determined by SDS-PAGE.
Supplied as 0.2 μm filtered solution in 50 mM Tris, 150 mM NaCl, pH7.5 with glycerol as protectant.
Contact us for customized product form or formulation.
This product is supplied and shipped with dry ice, please inquire the shipping cost.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
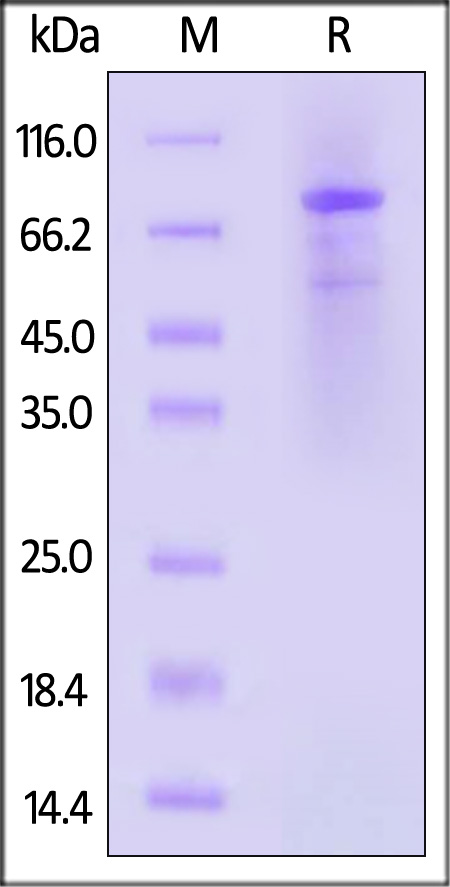
Human BTK Protein, His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90%.
The BTK assay is performed using the ADP-GloTM Kinase Assay kit which quantifies the amount of ADP produced by the BTK reaction. The ADP- GloTM Reagent is added to terminate the kinase reaction and to deplete the remaining ATP, and then the Kinase Detection Reagent is added to convert ADP to ATP and to measure the newly synthesized ATP using luciferase/luciferin reaction.The specific activity is >25 pmol/min/ug (QC tested).

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Orelabrutinib | ICP-022; BIIB-135 | Approved | Beijing Innocare Pharma Tech Co Ltd | 宜诺凯, INNOBRUKA, YINUOKAI | Mainland China | Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Beijing Innocare Pharma Tech Co Ltd | 2020-12-25 | Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma; Waldenstrom Macroglobulinemia; Lymphoma; Hepatic Insufficiency; Leukemia, B-Cell; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Helicobacter pylori infection; Lymphoma, B-Cell, Marginal Zone; Purpura, Thrombocytopenic, Idiopathic; Lymphoma, B-Cell | Details |
| Ibrutinib | PCI-32765; CRA-032765; JNJ-54179060; PCI-32765-00 | Approved | Pharmacyclics Llc | Imbruvica, 亿珂 | United States | Leukemia, Lymphocytic, Chronic, B-Cell | Pharmacyclics Inc | 2013-11-13 | Waldenstrom Macroglobulinemia; Monoclonal Gammopathy of Undetermined Significance; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Food Hypersensitivity; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Anemia, Aplastic; Prostatic Neoplasms; Leukemia, Prolymphocytic, B-Cell; Hepatic Insufficiency; Leukemia, B-Cell; Lymphoproliferative Disorders; Leukemia, Mast-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Neoplasms, Gonadal Tissue; Intraocular Lymphoma; Lung Neoplasms; Leukemia, Myeloid, Acute; Burkitt Lymphoma; Lymphoma, T-Cell; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Melanoma; Leukemia, Biphenotypic, Acute; Hepatitis B, Chronic; Leukemia; Skin Melanoma; Lymphoma, B-Cell, Marginal Zone; Immunoproliferative Small Intestinal Disease; Hematopoietic stem cell transplantation (HSCT); Leukemia, Myelogenous, Chronic; HIV Infections; Hematologic Neoplasms; Solid tumours; Carcinoid Tumor; Leukemia, Lymphoid; Esophageal Neoplasms; Lymphoma, B-Cell | Details |
| Acalabrutinib | ACP-196 | Approved | Astrazeneca Plc, Acerta Pharma Llc | Calquence, 康可期 | United States | Lymphoma, Mantle-Cell | Astrazeneca Plc | 2017-10-31 | Multiple Myeloma; Leukemia, Prolymphocytic; Carcinoma, Non-Small-Cell Lung; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Burkitt Lymphoma; Waldenstrom Macroglobulinemia; Lymphoma; Lymphoma, Non-Hodgkin; Hepatic Insufficiency; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Food Hypersensitivity; Richter's Syndrome; Anemia, Hemolytic, Autoimmune; Lymphoma, Mantle-Cell; Drug-Related Side Effects and Adverse Reactions; Pancreatic Neoplasms; Hodgkin Disease; Carcinoma, Transitional Cell; Arthritis, Rheumatoid; Graft vs Host Disease; Neoplasms; Glioblastoma; Coronavirus Disease 2019 (COVID-19); Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphoid; Squamous Cell Carcinoma of Head and Neck; Leukemia, Hairy Cell; Hematologic Neoplasms; Ovarian Neoplasms; Lymphoma, B-Cell | Details |
| Pirtobrutinib | LY 3527727; RXC-005; LOXO-305 | Approved | Redx Pharma Ltd | Jaypirca | United States | Lymphoma, Mantle-Cell | Loxo Oncology Inc | 2023-01-27 | Purpura, Thrombocytopenic, Idiopathic; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Hematologic Neoplasms; Leukemia; Leukemia, Lymphoid; Renal Insufficiency; Lymphoma, Large B-Cell, Diffuse; Multiple Sclerosis; Multiple Myeloma; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Hepatic Insufficiency; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Tirabrutinib hydrochloride | GS-4059; ONO-4059 | Approved | Ono Pharmaceutical Co Ltd | ベレキシブル, Velexbru | Japan | Lymphoma | Ono Pharmaceutical Co Ltd | 2020-03-25 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Sjogren's Syndrome; Chronic Urticaria; Arthritis, Rheumatoid; Lymphoma; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell; Pemphigus | Details |
| Zanubrutinib | BGB-3111 | Approved | BeOne Medicines Ltd | Brukinsa, 百悦泽 | United States | Lymphoma, Mantle-Cell | Beigene (Usa) Inc | 2019-11-14 | Lymphoma, Follicular; Carcinoma, Non-Small-Cell Lung; Melanoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphohistiocytosis, Hemophagocytic; Lymphoma, T-Cell; Lymphoma; Waldenstrom Macroglobulinemia; Hepatic Insufficiency; Leukemia, B-Cell; Anemia, Hemolytic, Autoimmune; Primary mediastinal B cell lymphoma; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Lung Diseases; Glomerulonephritis, Membranous; Lupus Nephritis; Neoplasms; Coronavirus Disease 2019 (COVID-19); Lymphoma, Large B-Cell, Diffuse; Immunoglobulin G4-Related Disease; Nephrosis; Leukemia; Solid tumours; Lymphoma, B-Cell, Marginal Zone | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Nemtabrutinib | ARQ-531; MK-1026 | Phase 3 Clinical | Arqule Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Richter's Syndrome; Hepatic Insufficiency; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Fenebrutinib | G-0853; RG-7845; GDC-0853; RO-7010939 | Phase 3 Clinical | Genentech Inc | Arthritis, Rheumatoid; Multiple Sclerosis; Multiple Sclerosis, Chronic Progressive; Lupus Erythematosus, Systemic; Urticaria; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Remibrutinib | LOU-064; LOU064-NXA; NVP-LOU064-NXA | Phase 3 Clinical | Novartis Pharma Ag | Multiple Sclerosis, Relapsing-Remitting; Myasthenia Gravis; Chronic Urticaria; Sjogren's Syndrome; Multiple Sclerosis; Dermatitis; Urticaria; Asthma; Hepatic Insufficiency; Sjogren-Larsson Syndrome; Peanut Hypersensitivity; Hidradenitis Suppurativa; Disease Susceptibility | Details |
| Larotinib Mesylate | Z-650 | Phase 3 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Pancreatic Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| Tolebrutinib | PRN-2246; SAR-442168 | Phase 3 Clinical | Principia Biopharma Inc | Myasthenia Gravis; Renal Insufficiency; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Hepatic Insufficiency | Details |
| Abivertinib | A-610MA; AC-0010; AC-0100; AC-001; AC-0010MA; AC-0100-Maleate; EX-ACEA0010MA | Phase 3 Clinical | Zhejiang Acea Pharmaceutical Co Ltd, Hangzhou Acea Pharmaceutical Research Co Ltd | Lymphoma, B-Cell; Coronavirus Disease 2019 (COVID-19); Lymphoma, Large B-Cell, Diffuse; Prostatic Neoplasms; Lymphoma, Mantle-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Atuzabrutinib | PRN-473; SAR-444727 | Phase 2 Clinical | Principia Biopharma Inc, Sanofi | Dermatitis, Atopic | Details |
| Elsubrutinib/Upadacitinib | ABBV-599 | Phase 2 Clinical | Abbvie Inc | Arthritis, Rheumatoid; Lupus Erythematosus, Systemic | Details |
| Poseltinib | HM-71224; LY3337641; NB-02 | Phase 2 Clinical | Hanmi Pharmaceutical Co Ltd | Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Central Nervous System Lymphoma; Primary vitreoretinal lymphoma | Details |
| TAS-5315 | TAS-5315 | Phase 2 Clinical | Taiho Pharmaceutical Co Ltd | Chronic Urticaria; Arthritis, Rheumatoid | Details |
| HZ-A-018 | HZ-A-018 | Phase 2 Clinical | Hangzhou Yuzheng Pharmaceutical Technology Co Ltd, HeaZen Therapeutics Co Ltd | Lymphoma, B-Cell; Multiple Sclerosis; Neuromyelitis Optica; Thrombocytopenia; Central Nervous System Lymphoma | Details |
| Branebrutinib | BMS-986195 | Phase 2 Clinical | Bristol-Myers Squibb Company | Arthritis, Rheumatoid; Sjogren's Syndrome; Immune System Diseases; Lupus Erythematosus, Systemic; Dermatitis, Atopic | Details |
| BIIB-091 | BIIB-091 | Phase 2 Clinical | Biogen Inc | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis | Details |
| WXFL10230486 | WXFL-10230486; WX-486; HWH-486 | Phase 2 Clinical | Wuxi Apptec Co Ltd, Humanwell Healthcare (Group) Co Ltd | Chronic Urticaria; Arthritis, Rheumatoid | Details |
| M-7583 | M7583; M-7583 | Phase 2 Clinical | Merck & Co Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Edralbrutinib | TG-1701; SHR-1459 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd, Jiangsu shengdi Medicine Co Ltd | Lymphoma, B-Cell; Nephrosis; Arthritis, Rheumatoid; Autoimmune Diseases; Glomerulonephritis, Membranous; Neuromyelitis Optica; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| TL-925 | TL-925 | Phase 2 Clinical | Telios Pharmaceuticals | Dry Eye Syndromes; Conjunctivitis, Allergic | Details |
| HBW-3210 | HBW-3210; HBW3210 | Phase 2 Clinical | Chengdu Haibowei Pharmaceutical Co Ltd | Lymphoma, B-Cell | Details |
| TQB-3702 | TQB3702; TQB-3702 | Phase 2 Clinical | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | Lymphoma, B-Cell; Hematologic Neoplasms; Neoplasms; Chronic Urticaria; Lupus Erythematosus, Systemic | Details |
| HBW-3220 | HBW-3220; HBW-3-10; HBW-003 | Phase 2 Clinical | Chengdu Haibowei Pharmaceutical Co Ltd | Lymphoma, B-Cell; Autoimmune Diseases; Lymphoma, Non-Hodgkin; Glomerulonephritis | Details |
| DZD-8586 | DZD-8586 | Phase 2 Clinical | Dizal (Jiangsu) Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BGB-16673 | BGB-16673; [14C]-BGB-16673 | Phase 2 Clinical | BeOne Medicines Ltd | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| HMPL-760 | HMPL-760; HMPL760 | Phase 2 Clinical | Hutchison Medipharma Ltd | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Richter's Syndrome; Lymphoma, Follicular; Lymphoma; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rocbrutinib | LP-168; NWP-775 | Phase 2 Clinical | Newave Pharmaceutical Inc | Lymphoma, B-Cell, Marginal Zone; Hematologic Neoplasms; Lymphoma, B-Cell; Leukemia, Hairy Cell; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Multiple Sclerosis; Neuromyelitis Optica; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| MH-048 | MH-048; MH048 | Phase 2 Clinical | Minghui Pharmaceutical (Shanghai) Co Ltd | Lymphoma, B-Cell | Details |
| Elsubrutinib | ABBV-105 | Phase 2 Clinical | Abbvie Ltd | Arthritis, Rheumatoid; Lupus Erythematosus, Systemic | Details |
| CX-1440 | CX1440; CX-1440 | Phase 2 Clinical | Hangzhou Sanyintai Pharmaceutical Technology Co Ltd, Hangzhou Aojin Biomedical Technology Co Ltd | Lymphoma, B-Cell; Purpura, Thrombocytopenic, Idiopathic; Graft vs Host Disease; Multiple Sclerosis; Lupus Erythematosus, Systemic; Urticaria; Leukemia, B-Cell; Thrombocytopenia | Details |
| JNJ-64264681 | JNJ-4681; JNJ-64264681 | Phase 1 Clinical | Johnson & Johnson | Solid tumours; Hematologic Neoplasms; Neoplasms; Myelodysplastic Syndromes; Prostatic Neoplasms, Castration-Resistant; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| PCI-45292 | Phase 1 Clinical | Pharmacyclics Llc | Arthritis, Rheumatoid; Autoimmune Diseases | Details | |
| BMS-935177 | Phase 1 Clinical | Bristol-Myers Squibb Company | Immune System Diseases | Details | |
| BT-1053 | BT-1053 | Phase 1 Clinical | Chengdu Brilliant Pharmaceutical Co Ltd, ScinnoHub Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| BTK-Max | BTK-Max | Phase 1 Clinical | Bristol-Myers Squibb Company | Arthritis, Rheumatoid; Psoriasis | Details |
| DTRMWXHS-12 | DTRMWXHS-12; DTRM-12 | Phase 1 Clinical | Zhejiang Dtrm Biopharma Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| FCN-647 | FCN-647 | Phase 1 Clinical | Fochon Pharmaceuticals Ltd | Lymphoma, B-Cell; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| SN-1011 | SN-1011 | Phase 1 Clinical | Sinomab Bioscience Ltd | Lupus Erythematosus, Systemic | Details |
| AC-0058TA | AC-0058; AC-0058TA | Phase 1 Clinical | Hangzhou Acea Pharmaceutical Research Co Ltd, Zhejiang Acea Pharmaceutical Co Ltd | Arthritis, Rheumatoid; Lupus Erythematosus, Systemic | Details |
| BI-BTK-1 | BI-BTK-1 | Phase 1 Clinical | C.H. Boehringer Sohn Ag & Co. Kg | Lupus Nephritis | Details |
| DTRM-505 | DTRM-505 | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| DWP-212525 | DWP-212525 | Phase 1 Clinical | Daewoong Pharmaceutical Co Ltd | Autoimmune Diseases; Arthritis, Rheumatoid; Lupus Erythematosus, Systemic; Pemphigus | Details |
| DWP-213388 | DWP-213388 | Phase 1 Clinical | Daewoong Pharmaceutical Co Ltd | Autoimmune Diseases | Details |
| NX-5948 | NX-5948 | Phase 1 Clinical | Nurix Therapeutics Inc | Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Waldenstrom Macroglobulinemia; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| UBX-303061 | UBX-303061 | Phase 1 Clinical | Ubix Therapeutics Inc | Lymphoma, B-Cell | Details |
| [14C]Pirtobrutinib | LOXO-305; [14C]RXC-005; RXC-005; [14C]LOXO-305 | Phase 1 Clinical | Redx Pharma Ltd | Details | |
| ANG-0623 | ANG-0623; ANG0623 | Phase 1 Clinical | Angel Pharmaceuticals Co Ltd | Multiple Sclerosis | Details |
| XS-04 | XS-04 | Phase 1 Clinical | Jiangsu Xingsheng Xinhui Pharmaceutical Co Ltd | Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse | Details |
| HZ-Q1070 | HZ-Q1070; HZ-Q-1070 | Phase 1 Clinical | HeaZen Therapeutics Co Ltd | Lymphoma, B-Cell; Neoplasms | Details |
| ABBV-101 | ABBV-101 | Phase 1 Clinical | Abbvie Inc | Hematologic Neoplasms | Details |
| IMG-004 | IMG-004 | Phase 1 Clinical | Hutchmed (China) Ltd | Chronic Urticaria | Details |
| XNW-1011 | EVER001; EVER-001; XNW1011; XNW-1011; SN-1011; SN1011 | Phase 1 Clinical | Suzhou Sinovent Pharmaceuticals Co Ltd, Sinomab Bioscience Ltd | Lymphoma, B-Cell; Autoimmune Diseases; Glomerulonephritis, Membranous; Multiple Sclerosis; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Nephrotic Syndrome; Pemphigus | Details |
| AC-0676 | AC0676; AC-676; AC676; AC-0676 | Phase 1 Clinical | Accutar Biotechnology Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Autoimmune Diseases | Details |
| JDB175 | JDB175 | Phase 1 Clinical | Shanghai Jiabao Yaoyin Pharmaceutical Technology Co Ltd | Lymphoma, Large B-Cell, Diffuse; Multiple Sclerosis | Details |
| SS-001 | SS-001 | Phase 1 Clinical | Zibo Baijichangsheng Pharmaceutical Co Ltd | Lymphoma, B-Cell | Details |
| BMS-986196 | 11C-BMS-986196 | Phase 1 Clinical | Bristol-Myers Squibb Company | Multiple Sclerosis | Details |
| Docirbrutinib | AS-1763; BN-102; CB-1763 | Phase 1 Clinical | Bionova Pharmaceuticals (Shanghai) Ltd, Carna Biosciences Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Sofnobrutinib | AS-0871; AS-871 | Phase 1 Clinical | Carna Biosciences Inc | Autoimmune Diseases | Details |
| NX-2127 | NX-2127 | Phase 1 Clinical | Nurix Therapeutics Inc | Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details |
| TT-01488 | TT-01488 | Phase 1 Clinical | TransThera Sciences (Nanjing) Inc | Lymphoma, B-Cell; Solid tumours; Leukemia, B-Cell | Details |
| SYHA-1811 | SYHA-1811 | Phase 1 Clinical | Shanghai Runshi Pharmaceutical Technology Co Ltd | Lymphoma, B-Cell | Details |
| HSK-29116 | HSK-29116 | Phase 1 Clinical | Sichuan Haisco Pharmaceutical Co Ltd | Lymphoma, B-Cell | Details |
| YZJ-3058 | Phase 1 Clinical | Shanghai Haiyan Pharmaceutical Technology Co Ltd | Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| ZXBT-1158 | ZXBT-1158 | Phase 1 Clinical | Guangzhou BeBetter Medicine Technology Co | Lymphoma, B-Cell; Multiple Sclerosis | Details |
| Luxeptinib | CG026806; CG-806; CG’806; CG-026806 | Phase 1 Clinical | Crystalgenomics Inc | Hematologic Neoplasms; Myelodysplastic Syndromes; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Evobrutinib | M-2951; MSC-2364447; MSC-2364447-C; MSC2364447C | Phase 1 Clinical | Merck Serono | Multiple Sclerosis, Relapsing-Remitting; Renal Insufficiency; Arthritis, Rheumatoid; Multiple Sclerosis; Lupus Erythematosus, Systemic; Hepatic Insufficiency | Details |
| GB-5121 | GB-5121; GB5121 | Clinical | Gossamer Bio Inc | Neurolymphomatosis; Central Nervous System Lymphoma | Details |
This web search service is supported by Google Inc.







