
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































This protein carries flag tag at the N-terminus and polyhistidine tag at the C-terminus.
The protein has a calculated MW of 58.4 kDa.
>90% as determined by SDS-PAGE.
This product is not suitable for cell based experiments due to cytotoxicity of detergent.
Detergent buffer is INDISPENSABLE to keep membrane protein soluble and active, under no circumastance should you remove detergent.
Detergent buffer is sold separately and not included in protein, and please contact us if you need the buffer.
If glycerol is not compatible to your application, remove glycerol just before immediate experiment, and NEVER store glycerol-free protein solution.
Supplied as 0.2 μm filtered solution in 50 mM HEPES, 150 mM NaCl, Buffer B, pH7.5 with glycerol as protectant.
Contact us for customized product form or formulation.
This product is supplied and shipped with dry ice, please inquire the shipping cost.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
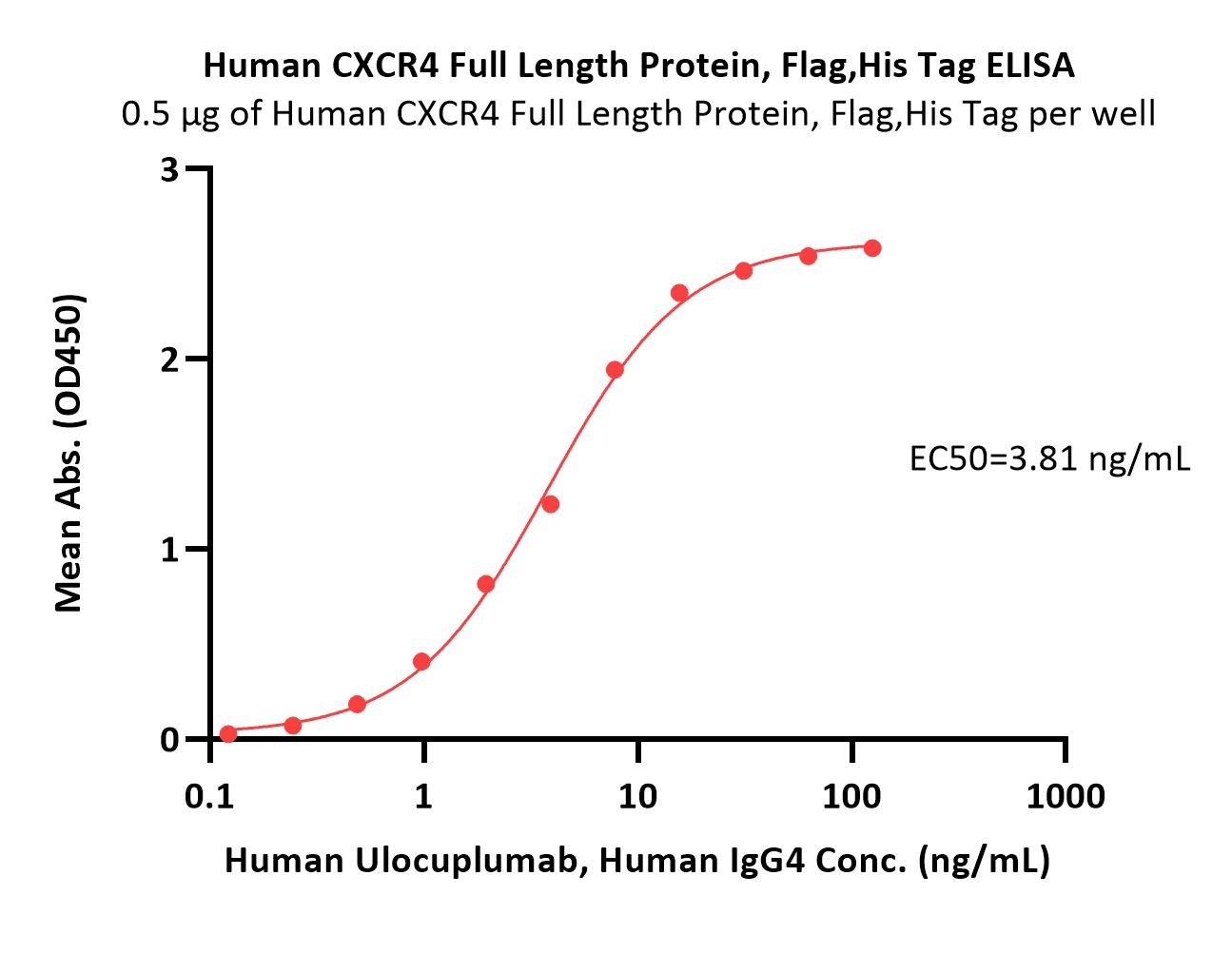
Immobilized Human CXCR4 Full Length Protein, Flag,His Tag (Cat. No. CX4-H52D3) at 5 μg/mL (100 μL/well) can bind Human Ulocuplumab, Human IgG4 with a linear range of 0.1-16 ng/mL (QC tested).
Price(USD) : $805.00
Price(USD) : $2985.00
Price(USD) : $7480.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Plerixafor | SDZ-SID-791; JM-3100; JKL-169; AMD-3100; PLR-001; LM-3100 | Approved | Genzyme Corp | Mozobil, Mobozil, 释倍灵 | United States | Lymphoma, Non-Hodgkin; Multiple Myeloma | Genzyme Corp | 2008-12-15 | Agranulocytosis; Pulmonary Fibrosis; Rejection of organ transplantation; Respiratory Distress Syndrome, Adult; Leukopenia; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Osteosarcoma; Colorectal Neoplasms; Lymphopenia; Multiple Myeloma; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Pulmonary Disease, Chronic Obstructive; Lymphoma; Granulomatous Disease, Chronic; Adenocarcinoma; Leukemia, Lymphocytic, Chronic, B-Cell; Anemia, Sickle Cell; WHIM syndrome; Hematopoietic stem cell transplantation (HSCT); Cystic Fibrosis; Infections; Leukemia, Myelogenous, Chronic; Wiskott-Aldrich Syndrome; Ovarian Neoplasms; Hematologic Neoplasms; Neutropenia; Bone metastases; Warts; Primary Graft Dysfunction; Coronavirus Disease 2019 (COVID-19); Fanconi Anemia; Hodgkin Disease; Pancreatic Neoplasms; Myelodysplastic Syndromes | Details |
| Mavorixafor | AMD-11070; AMD-070; X4P-001; X4P-001-RD; X4P-001-LD; ABSK-081; X4P-001-IO | Approved | Genzyme Corp | XOLREMDI, Xolremdi | United States | WHIM syndrome | X4 Pharmaceuticals Inc | 2024-04-26 | HIV Infections; WHIM syndrome; Neutropenia; Carcinoma, Renal Cell; Triple Negative Breast Neoplasms; Waldenstrom Macroglobulinemia; Melanoma | Details |
| Motixafortide | 4F-Benzoyl-TN14003; TN-14003; TF-14016; BL-8040; BKT-140; GFH-168; 4F-benzoyl-TN-14003 | Approved | Biokine, Kyoto University | APHEXDA | United States | Hematopoietic stem cell transplantation (HSCT) | Biolinerx Ltd | 2023-09-08 | Leukemia, Myelogenous, Chronic; Hematopoietic stem cell transplantation (HSCT); Esophageal Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Hodgkin Disease; Pancreatic Neoplasms; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Anemia, Aplastic; Leukemia, Myeloid, Acute; Lymphoma, T-Cell; Lymphoma, Non-Hodgkin; Anemia, Sickle Cell; Adenocarcinoma | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| [68Ga]Pentixafor | [68Ga]Ga-PentixaFor; CXCR4-PET; 68Ga-PTF; [68Ga]Ga-PentixaFor PET/CT; [68Ga]Ga-PTF | Phase 3 Clinical | The University Of Iowa, Pentixapharm AG | Lymphoma, B-Cell, Marginal Zone; Cushing Syndrome; Sarcoidosis; Rejection of organ transplantation; Neuroendocrine Tumors; Multiple Myeloma; Histiocytosis, Sinus; Erdheim-Chester Disease; Histiocytic Sarcoma; Myocarditis; Lymphoma, Non-Hodgkin; Hyperaldosteronism; Central Nervous System Lymphoma | Details |
| 90Y-Pentixather | Phase 2 Clinical | University Of Wurzburg, Technical University Of Munich | Multiple Myeloma; Central Nervous System Lymphoma | Details | |
| 177Lu-Pentixather | Phase 2 Clinical | Technical University Of Munich, Nantes University Hospital, University Of Wurzburg | Leukemia; Multiple Myeloma | Details | |
| Burixafor Hydrobromide | TG-0054; GP 01; GPC-100; TG-3000 | Phase 2 Clinical | TaiGen Biotechnology Co Ltd | Hematopoietic stem cell transplantation (HSCT); Hodgkin Disease; Multiple Myeloma; Prostatic Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| MSX-122 | Q-122; WZ-40; MSX-122 | Phase 2 Clinical | Altiris Therapeutics | Solid tumours; Hot Flashes | Details |
| PTX-9908 | PTX-9908 | Phase 2 Clinical | Pertinax Therapeutics | Carcinoma, Hepatocellular | Details |
| Ga-68-CXCR4 | Ga-68-CXCR4 | Phase 2 Clinical | Koo Foundation Sun Yat-Sen Cancer Center | Lymphoma, B-Cell | Details |
| Plerixafor/Tacrolimus Hydrate | AMD3100+FK506; MRG-001(MedRegen) | Phase 2 Clinical | MedRegen LLC | Motor Neuron Disease; Cytokine Release Syndrome; Hepatitis, Alcoholic; Respiratory Tract Diseases; Coronavirus Disease 2019 (COVID-19); Respiratory Distress Syndrome, Adult; Abdominal Injuries; Wounds and Injuries; Respiratory Insufficiency; Amyotrophic Lateral Sclerosis | Details |
| NRP-2945 | NRP-2945; NNZ-4945 | Phase 2 Clinical | Neuren Pharmaceuticals Ltd, Calzada Ltd | Angelman Syndrome; Rett Syndrome; Lennox Gastaut Syndrome; Epilepsy, Absence; Epilepsy, Temporal Lobe; Amyotrophic Lateral Sclerosis | Details |
| 4-P-021 | 4P021; 4-P-021 | Phase 2 Clinical | 4P-Pharma Ltd, University of Bordeaux, Institut Pasteur De Lille | Coronavirus Disease 2019 (COVID-19) | Details |
| CGT-1881 | CGT1881; CGT-1881 | Phase 2 Clinical | Cgenetech(Suzhou China) Co Ltd | Hematopoietic stem cell transplantation (HSCT); Hematologic Neoplasms; Multiple Myeloma; Lymphoma, Non-Hodgkin | Details |
| AGT 103-T | AGT 103-T | Phase 1 Clinical | American Gene Technologies International Inc | HIV Infections | Details |
| Balixafortide | POL-6326 | Phase 1 Clinical | Polyphor Ltd | Leukemia, Myelogenous, Chronic; Myelodysplastic Syndromes; Myeloproliferative Disorders; Multiple Myeloma; Breast Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Metastatic breast cancer; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| JVS-100 | JVS-100; SRX-100; ACRX-100; GP-51801; GP51801 | Phase 1 Clinical | Cleveland Clinic | Heart Failure; Ischemia; Surgical Wound; Peripheral Arterial Disease | Details |
| 68Ga-JH12 | 68Ga-JH12 | Phase 1 Clinical | First Affiliated Hospital Of Fujian Medical University | Neoplasms | Details |
| 203-Lead Pentixather | Phase 1 Clinical | National Institutes Of Health, National Cancer Institute, Holden Comprehensive Cancer Center | Small Cell Lung Carcinoma; Lung Neoplasms | Details | |
| 212-Lead Pentixather | Phase 1 Clinical | National Institutes Of Health, National Cancer Institute, Holden Comprehensive Cancer Center | Small Cell Lung Carcinoma; Lung Neoplasms | Details | |
| 68Ga-Pentixather | 68Ga-Pentixather | Phase 1 Clinical | Peking Union Medical College Hospital | Multiple Myeloma | Details |
| CXCR4 modified anti-BCMA CAR T cell therapy (Sichuan University) | Phase 1 Clinical | Sichuan University | Multiple Myeloma | Details | |
| AD-214 | AD-214 | Phase 1 Clinical | Adalta Ltd | Lung Diseases, Interstitial | Details |
| X-4136 | X-4136 | Clinical | X4 Pharmaceuticals Inc | Lymphoma, B-Cell; Melanoma; Uterine Cervical Neoplasms | Details |
| [18F]AlF-NOTA-pentixather | Sichuan Provincial People'S Hospital | Details |
This web search service is supported by Google Inc.







