
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































> Insights > Soluble B-cell maturation antigen (sBCMA) plays a dual role in targeted immunotherapy B-cell maturation antigen (BCMA), encoded by the TNFRSF17 gene located on the short arm of chromosome 16, is a type III transmembrane glycoprotein predominantly expressed on the surface of plasma cells and myeloma cells. Under the action of specific enzymes, such as γ-secretase, the extracellular domain of BCMA is cleaved and released into the bloodstream as soluble BCMA (sBCMA). Additionally, sBCMA may also shed from the cell surface into the bloodstream via extracellular vesicles, such as exosomes. In diseases like multiple myeloma (MM), the high proliferation and metabolic activity of tumor cells significantly increase the expression and shedding of BCMA, leading to elevated sBCMA levels. These mechanisms collectively result in the accumulation of sBCMA in the blood, making it a crucial indicator for disease monitoring and treatment.
Early studies have shown that anti-BCMA antibodies exhibit potent cytotoxic effects on MM cells in vitro. Currently, various innovative BCMA-targeted immunotherapeutic agents are under clinical development, including CAR-T cells, T-cell engagers (TCEs), and antibody-drug conjugates (ADCs). The mechanisms of these agents are as follows:
• ADC: After binding to BCMA on the surface of MM cells, ADCs are internalized. The linker is hydrolyzed in lysosomes or endosomes, releasing cytotoxic drugs that induce tumor cell death.
• CAR-T Cells: The single-chain variable fragment (scFv) on CAR-T cells binds to BCMA on MM cells, activating CAR-T cells and releasing cytotoxic cytokines to kill tumor cells.
• TCEs: By simultaneously binding to CD3 on T cells and BCMA on MM cells, TCEs promote the crosslinking of T cells with tumor cells, activating T cells and releasing cytotoxic factors to induce tumor cell death.
sBCMA is a significant biomarker in MM treatment, with its levels directly correlating with disease burden. Numerous studies have shown:
• Elevated sBCMA levels are associated with active MM and high tumor burden.
• A decrease in sBCMA levels during treatment typically indicates a good response, while an increase may suggest relapse or progression.
• Sustained low sBCMA levels during maintenance therapy indicate long-term disease control, whereas higher baseline levels are associated with poorer prognosis.
Furthermore, changes in sBCMA levels can serve as quantitative indicators for BCMA-targeted immunotherapy: a decrease in sBCMA suggests effective treatment, while an increase often precedes clinical or imaging evidence of relapse, aiding in early intervention.
Although sBCMA is an important biomarker, high levels may interfere with the efficacy of BCMA-targeted therapies through competitive binding:
• Reduced Drug Binding Efficiency: High concentrations of sBCMA competitively bind to targeted drugs, reducing their binding opportunities with tumor cells. For example, sBCMA may sequester ADCs, decreasing their efficiency in delivering cytotoxic drugs to tumor cells.
• Blocking Immune Activation: sBCMA may obstruct key interactions necessary for T-cell activation and tumor cell killing. Excessive sBCMA can saturate TCEs or CAR-T cells, weakening their ability to connect T cells with tumor cells, thereby reducing antitumor effects.
• Affecting Pharmacokinetics: Binding of sBCMA to therapeutic antibodies may accelerate drug clearance, shorten half-life, and alter distribution, leading to insufficient efficacy.
sBCMA plays a dual role in MM treatment: on one hand, it is a crucial biomarker for disease monitoring and treatment response evaluation; on the other hand, its high levels may interfere with the efficacy of BCMA-targeted immunotherapy through a decoy effect. Future strategies should focus on optimizing drug design, developing adjunctive therapies, or exploring alternative targets to overcome the negative impact of sBCMA, thereby enhancing treatment outcomes.
To accelerate the clinical development of BCMA-targeted drugs, ACROBiosystems has launched the ClinMax™ Human Soluble BCMA ELISA Kit, empowering clinical research with three core advantages:
• Real Sample Validation: Tested with various real samples to ensure accurate and reliable results.
• Matrix Interference Optimization: Overcomes the limitations of complex biological matrices, ensuring experimental stability and reproducibility.
• Ready-to-Use Design: Pre-coated plates and standardized procedures save time and improve efficiency, allowing for immediate experimentation.
ACROBiosystems simplifies the research and development process with innovative technology, enhancing data quality and drug development efficiency, and safeguarding breakthroughs in BCMA-targeted therapies!
Additionally, we offer an sBCMA antibody library and support customized development services for Free/Total sBCMA ELISA kits. For inquiries, please email service.cn@acrobiosystems.com or call our customer service at 010-53681107.
ClinMax™ Human Soluble BCMA ELISA Kit (CEA-B045)
Linear Range
The linear detection range of the ClinMax™ Human Soluble BCMA ELISA Kit is 375 pg/mL – 24,000 pg/mL, as shown in Figure 1.
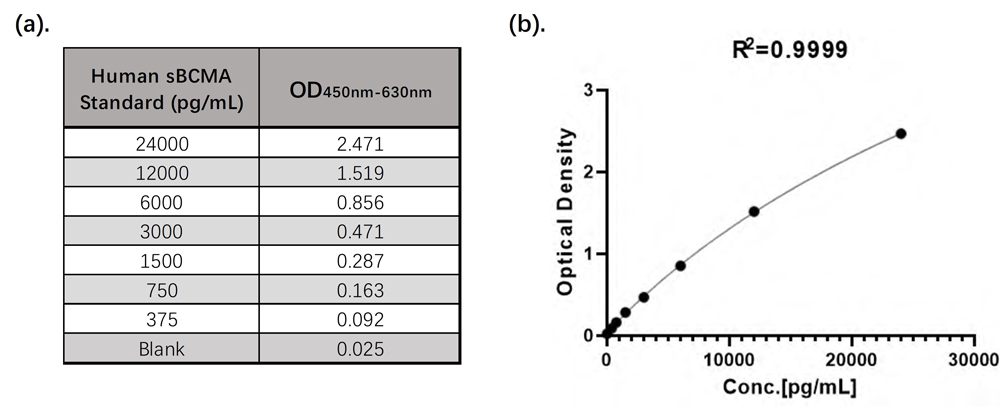
Figure 1. (a). CEA-B045 Linearity Range Data; (b). CEA-B045 4PL Standard Curve
Sample Values
30 healthy serum samples were evaluated for the concentrations of human sBCMA in assay, as shown in Figure 2.
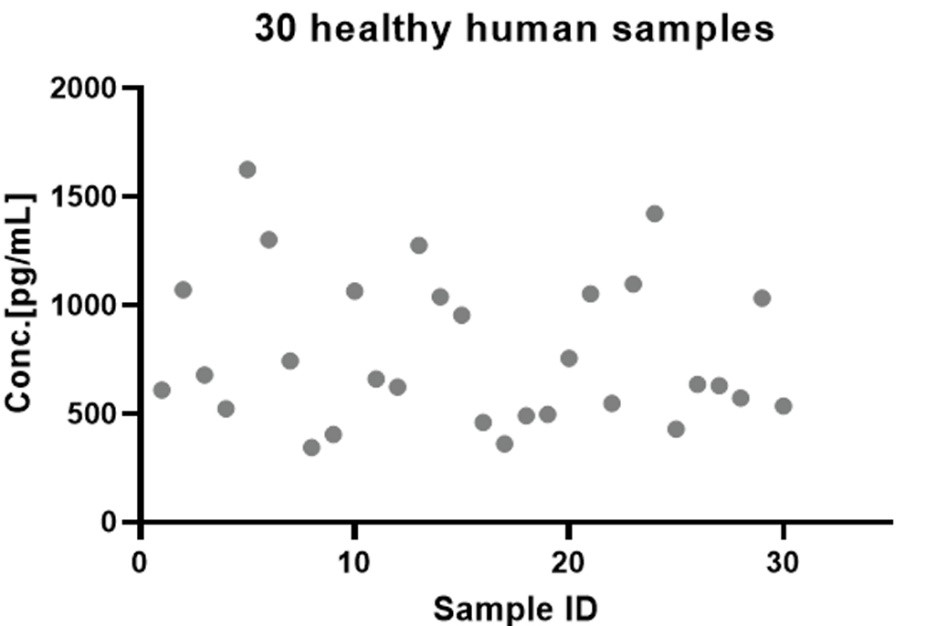
Figure 2. Quantification of sBCMA in Human Serum via CEA-B045 ELISA Kit
This web search service is supported by Google Inc.







