
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































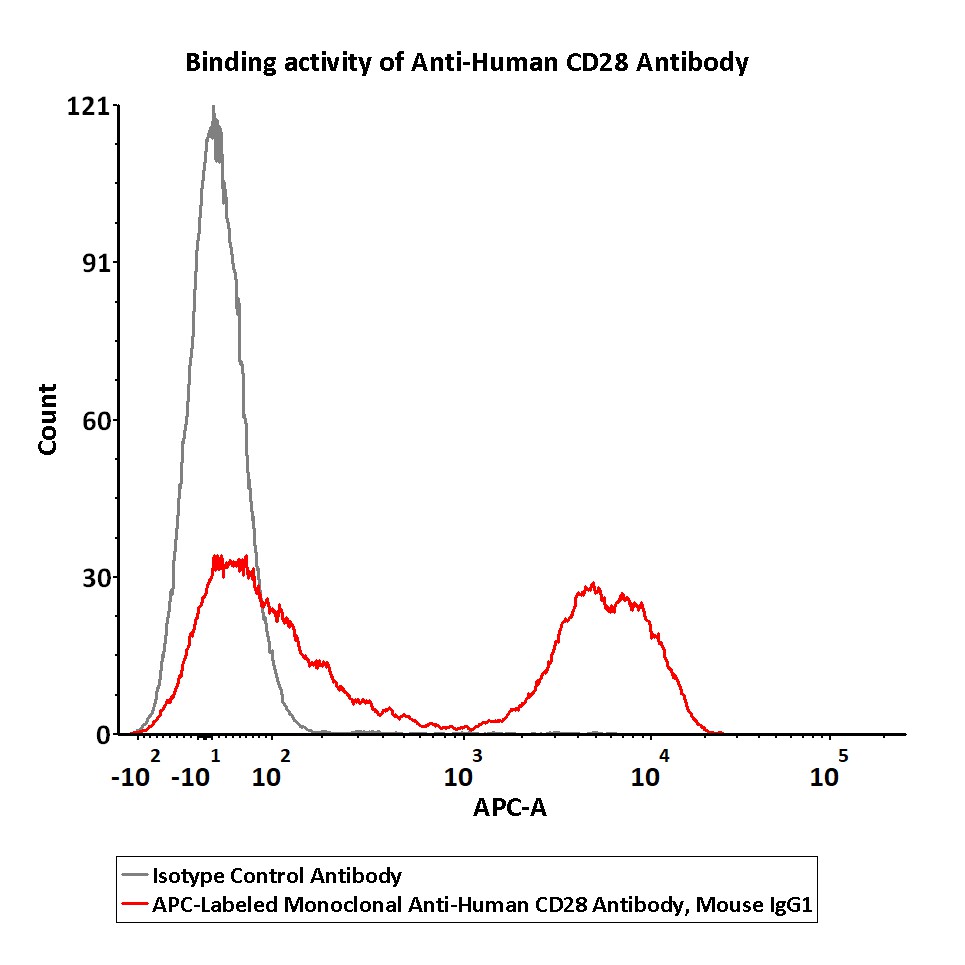
Flow cytometric analysis of Human peripheral blood lymphocytes respectively staining with APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (Cat. No. CD8-AHFC1) at 1:50 dilution (2 μL of the antibody stock solution corresponds to labeling of 1e6 cells in a final volume of 100 µL), compared with isotype control antibody. APC signal was used to evaluate the binding activity (QC tested).
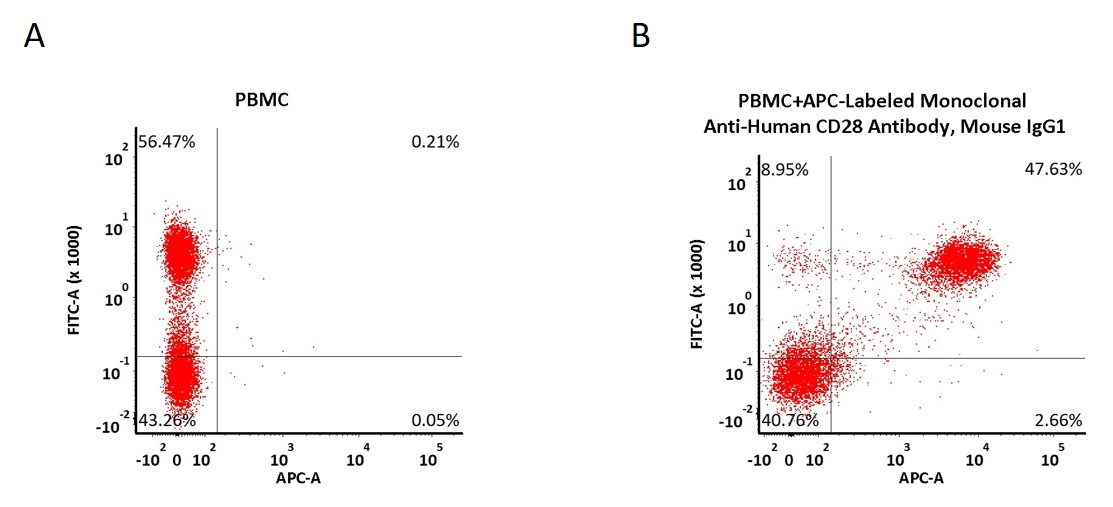
Non-specificity of APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (Cat. No. CD8-AHFC1) binding to CD3- cells present in human PBMC. Human PBMCs were simultaneously stained with FITC anti-human CD3 Antibody and APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (2 μL of the antibody stock solution corresponds to labeling of 5e5 cells in a final volume of 100 µL), washed and then analyzed with FACS. FITC negative signals and APC positive signals were used to evaluate the non-specific binding activity to human CD3- cells (Routinely tested).
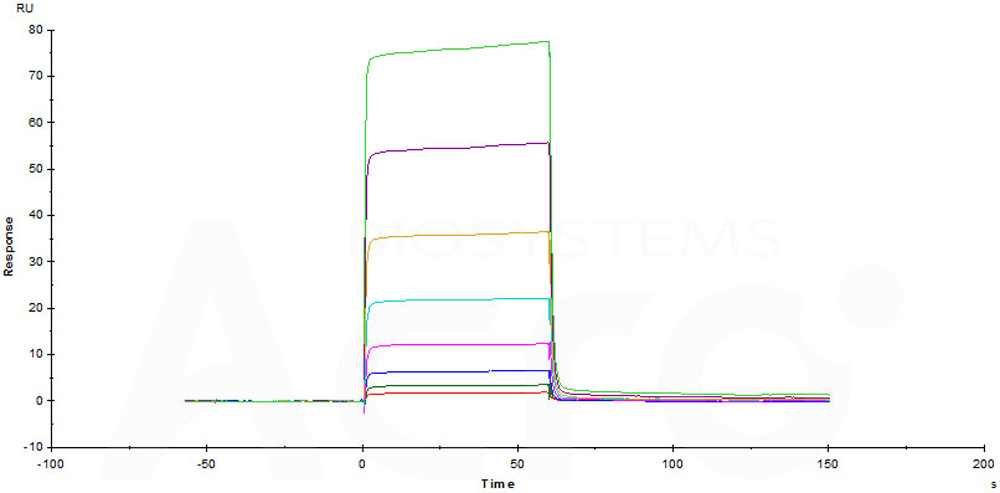
Immobilized Human / Cynomolgus / Rhesus macaque CD28, Fc Tag (Cat. No. CD8-H525a) on CM5 Chip can bind Human B7-1, His Tag (Cat. No. B71-H5228) with an affinity constant of 5.29 μM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Nezastomig | REGN-5678 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| Abatacept (Orban Biotech) | Phase 2 Clinical | Orban Biotech, The National Institute Of Diabetes And Digestive And Kidney Diseases | Diabetes Mellitus, Type 1 | Details | |
| Lulizumab pegol | Lulizumab; BMS-931699 | Phase 2 Clinical | Bristol-Myers Squibb Company | Rejection of renal transplantation; Lupus Vulgaris; Sjogren-Larsson Syndrome | Details |
| Acazicolcept | ALPN-101 | Phase 2 Clinical | Alpine Immune Sciences Inc | Autoimmune Diseases; Graft vs Host Disease; Lupus Erythematosus, Systemic; Lymphoproliferative Disorders; Inflammation | Details |
| FR-104 | FR-104; JNJ-3133; VEL-101 | Phase 2 Clinical | Effimune | Rejection of renal transplantation; Arthritis, Rheumatoid; Postoperative Complications | Details |
| TC-510 | TC-510 | Phase 2 Clinical | Tcr2 Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| REGN-7945 | REGN7945; REGN-7945 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Multiple Myeloma | Details |
| MDX-2001 | MDX-2001; MDX2001 | Phase 2 Clinical | ModeX Therapeutics Inc | Kidney Neoplasms; Biliary Tract Neoplasms; Head and Neck Neoplasms; Esophageal Neoplasms; Rectal Neoplasms; Stomach Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Thyroid Neoplasms; Endometrial Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms | Details |
| REGN-7075 | REGN-7075 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details |
| REGN-5668 | REGN-5668 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Ovarian Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Endometrial Neoplasms | Details |
| ATLCAR.k.CD28 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 1 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details | |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| Autologous CD19CAR-CD28-CD3zeta-EGFRt-expressing Tcm-enriched T cells (City of Hope Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center | Lymphoma, Non-Hodgkin | Details | |
| SAR-446422 | SAR-446422; SAR446422 | Phase 1 Clinical | Sanofi | Autoimmune Diseases | Details |
| JNJ-1493 | JNJ-1493 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Lymphoma, B-Cell | Details |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| REGN5837 | REGN5837; REGN-5837 | Phase 1 Clinical | Regeneron Pharmaceuticals Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| XmAb-808 | XmAb808 | Phase 1 Clinical | Xencor Inc | Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| CD19 PD-1/CD28 CAR-T Cell Therapy (Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Primary mediastinal B cell lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Follicular | Details | |
| B7-2/GM-CSF cancer gene therapy | CIT | Phase 1 Clinical | Radient | Neoplasms | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
This web search service is supported by Google Inc.







