
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































This protein carries no "tag".
The protein has a calculated MW of 58.9 kDa. The protein migrates as 76 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
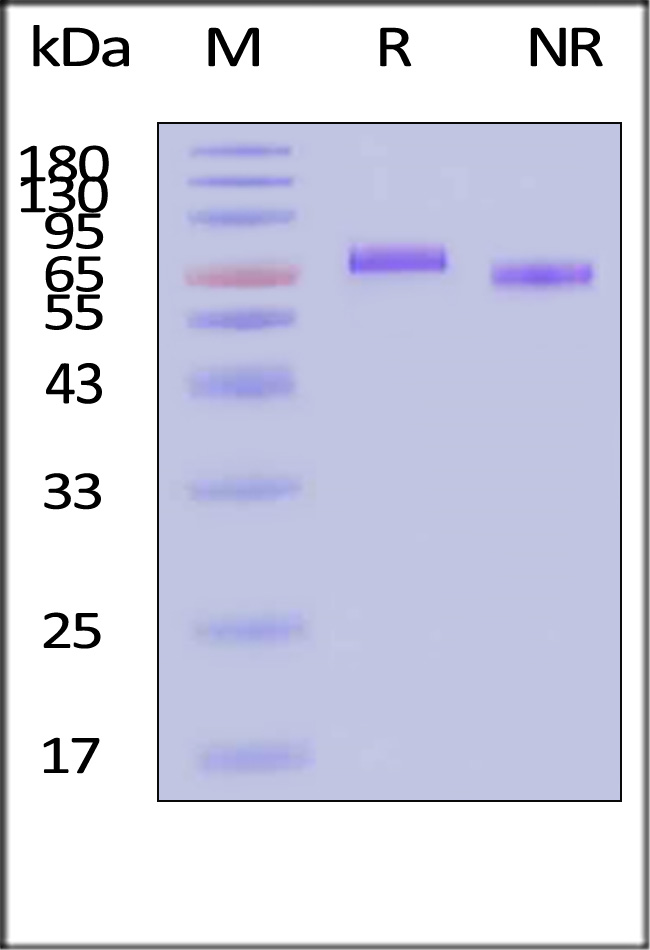
Human IL-12 Protein, premium grade on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).

The purity of Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) is more than 90% and the molecular weight of this protein is around 60-80 kDa verified by SEC-MALS.
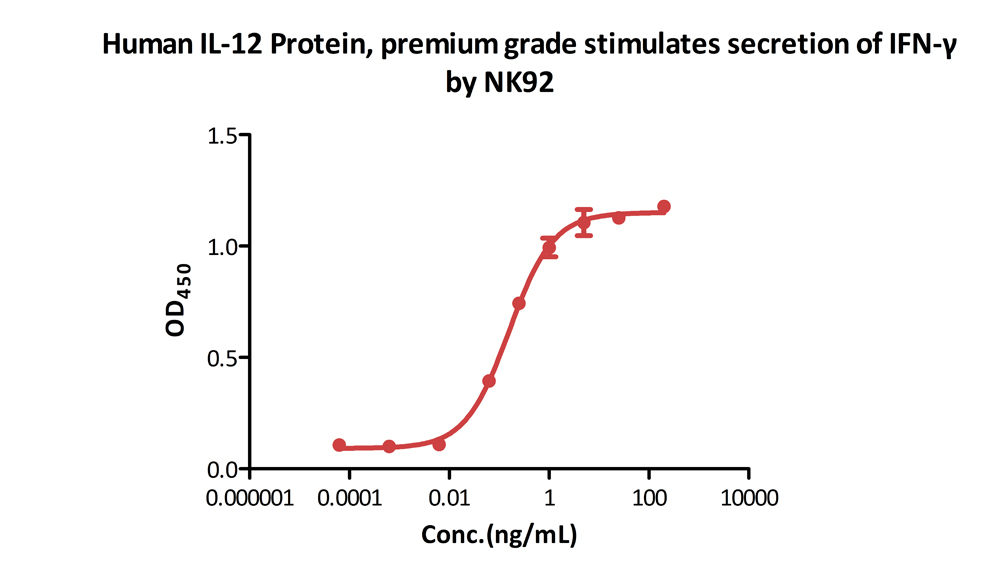
Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) stimulates secretion of IFN-γ by NK-92 human natural killer lymphoma cells. The specific activity of Human IL-12 Protein, premium grade is > 1.00ⅹ10^7 IU/mg, which is calibrated against human IL-12 WHO International Standard (NIBSC code: 95/544) (QC tested).
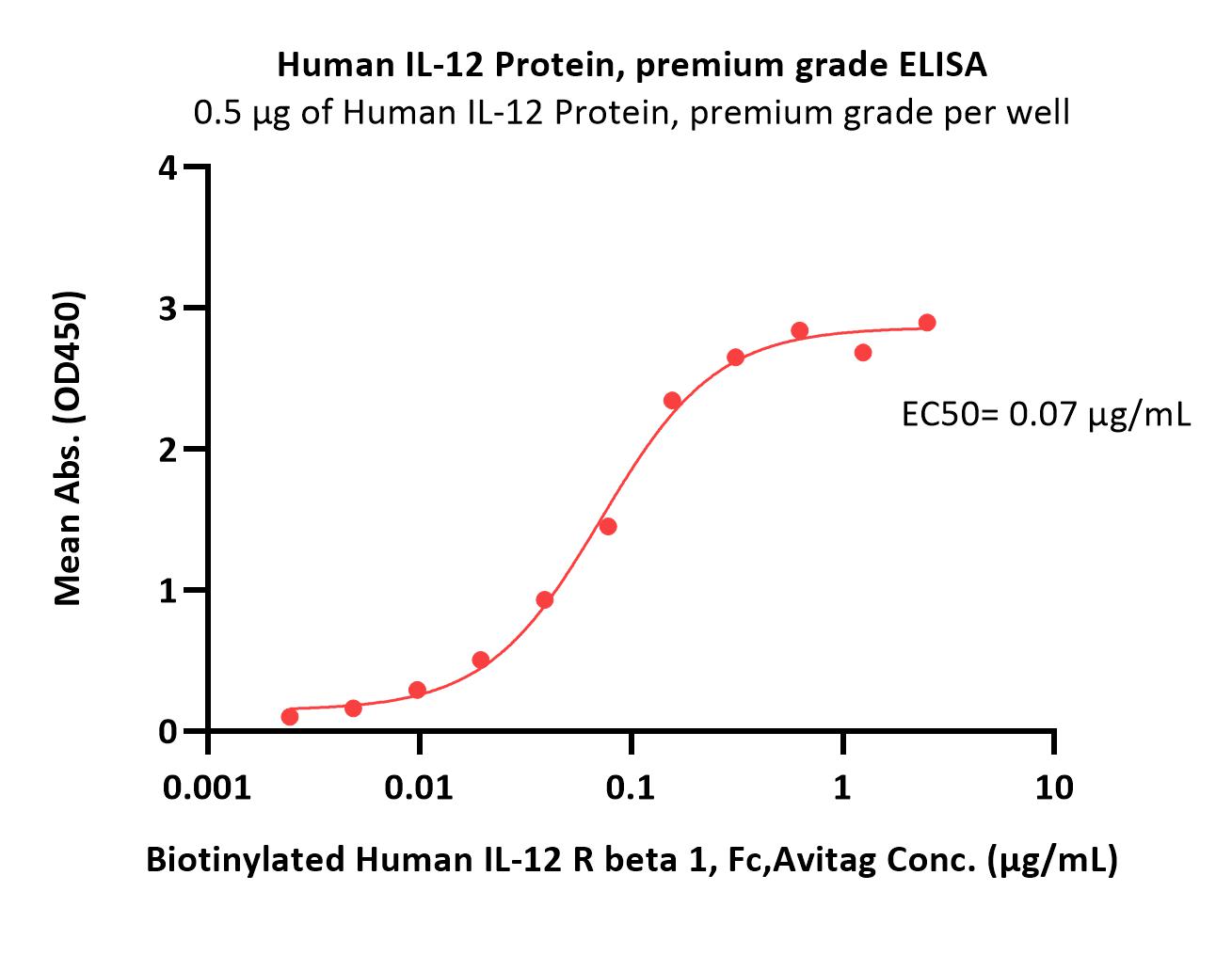
Immobilized Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) at 5 μg/mL (100 μL/well) can bind Biotinylated Human IL-12 R beta 1, Fc,Avitag (Cat. No. ILB-H82F7) with a linear range of 0.002-0.156 μg/mL (QC tested).

Immobilized Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Human IL23A&IL12B P40 domain Antibody, Human IgG1 with a linear range of 0.05-3 ng/mL (Routinely tested).
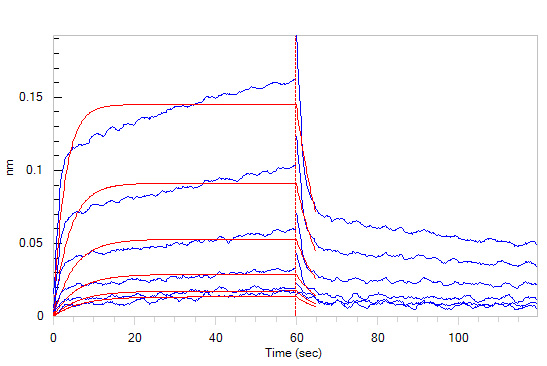
Loaded Biotinylated Human IL-12 R beta 1, Fc,Avitag (Cat. No. ILB-H82F7) on SA Biosensor, can bind Human IL-12 Protein, premium grade (Cat. No. IL2-H5210) with an affinity constant of 2.6 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).

The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG IL-12.
Price(USD) : $310.00
Price(USD) : $495.00
Price(USD) : $3605.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | C-340; CNTO-1275 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Multiple Sclerosis; Plaque psoriasis; Dermatitis, Atopic; Crohn Disease; Hidradenitis Suppurativa; Common Variable Immunodeficiency; Uveitis; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Lupus Erythematosus, Systemic; Psoriasis; Diabetes Mellitus, Type 1; Arthritis, Juvenile; Sjogren's Syndrome; Spondylitis, Ankylosing; Pouchitis; Ichthyosis; Dermatomyositis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Polymyositis; Liver Cirrhosis, Biliary | Details |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Approved | Formycon AG | OTULFI | EU | Crohn Disease; Colitis, Ulcerative | Fresenius Kabi Deutschland Gmbh, Formycon AG | 2024-09-25 | Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab Biosimilar (qyuns) | QX-001S; QX-001-S; HDM-3001 | Approved | Qyuns Therapeutics Co Ltd | Mainland China | Plaque psoriasis | Qyuns Therapeutics Co Ltd | 2024-10-29 | Psoriasis; Plaque psoriasis | Details | |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Approved | Celltrion Inc | Steqeyma, Steqeyma IV | Canada | Plaque psoriasis; Arthritis, Psoriatic; Crohn Disease | Celltrion Inc | 2024-08-01 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar (Biocon) | Approved | Biocon Biologics Ltd | Bmab 1200, Yesintek | United States | Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease | Biocon Biologics Inc | 2024-11-29 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details | |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™, JamtekiTM, Ustekinumab BS (F) | Japan | Arthritis, Psoriatic; Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA, wezenla | United States | Crohn Disease; Colitis, Ulcerative; Arthritis, Psoriatic; Plaque psoriasis | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | Xerostomia; Colorectal Neoplasms; Neuroectodermal Tumors, Primitive; Prostatitis; Primary Myelofibrosis; Osteosarcoma; Cholangitis, Sclerosing; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Adenocarcinoma, Clear Cell; Carcinoma, Adenosquamous; Epilepsy; Erythema Nodosum; Sarcoma; HIV Wasting Syndrome; Pancreatitis, Chronic; Sarcoma, Ewing; Arachnoiditis; Gastrointestinal Hemorrhage; Retinoblastoma; Prostatic Neoplasms; Endometriosis; Lung Neoplasms; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Myelodysplastic-Myeloproliferative Diseases; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Carcinoma, Hepatocellular; Burning Mouth Syndrome; Stomatitis; Neoplasm Metastasis; Appendiceal Neoplasms; Tuberculosis; Lymphoma, Non-Hodgkin; Angiodysplasia; Pelvic Pain; Glioma; Waldenstrom Macroglobulinemia; Leprosy, Lepromatous; Endometrial Neoplasms; Uterine Neoplasms; Anemia, Sideroblastic; Ovarian Neoplasms; Gastric Antral Vascular Ectasia; Lupus Erythematosus, Discoid; Carc | Details |
| Ustekinumab biosimilar(DM Bio) | DA-3115; DMB-3115 | Approved | Dong-A Pharmaceutical Co Ltd, Meiji Seika Pharma Co Ltd | IMULDOSA, Absimky | United States | Psoriasis | Dong-A ST Co Ltd, Meiji Seika Kaisha Ltd | 2024-10-11 | Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Lisofylline | BL-194; CT-1501R; A-802710 | Phase 3 Clinical | Cti Biopharma Corp | Diabetes Mellitus, Type 1; Rejection of organ transplantation | Details |
| Apilimod mesilate | AIT-101; STA-5326-mesylate; LAM-002; STA-5326; LAM-002A | Phase 2 Clinical | Madrigal Pharmaceuticals Inc, Lam | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Common Variable Immunodeficiency; Crohn Disease; Amyotrophic Lateral Sclerosis | Details |
| NSC-733972 | M-032; M032-H SV-1; NSC-733972 | Phase 2 Clinical | University Of Alabama At Birmingham | Glioblastoma; Gliosarcoma; Astrocytoma | Details |
| Tavokinogene telseplasmid | DNA IL-12; pIL-12 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | HIV Infections; Hemorrhagic Fever, Ebola; Carcinoma, Merkel Cell; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Prostatic Neoplasms; Hepatitis B; Lymphoma, T-Cell, Cutaneous; Melanoma; Mycosis Fungoides | Details |
| T-3011 | T-3 (ImmVira Pharma); MVR-T3011; T3011; MVR-T3011 IT; MVR-T3011 IV; B015; B-015 | Phase 2 Clinical | Immvira Co Ltd | Sarcoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Melanoma; Endometrial Neoplasms; Lung Neoplasms; Lymphoma; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Solid tumours; Urinary Bladder Neoplasms; Mesothelioma; Ascites; Skin Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Ovarian Neoplasms; Liver Neoplasms; Head and Neck Neoplasms | Details |
| PDS-0301 | M-9241; MSB-0010360; MSB0010360; PDS-0301 | Phase 2 Clinical | Emd Serono Inc, Merck Serono, National Cancer Institute | Prostatic Neoplasms; Sarcoma, Kaposi; Uterine Cervical Neoplasms; Urogenital Neoplasms; Metastatic breast cancer; Penile Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Cholangiocarcinoma; Vulvar Neoplasms; Breast Neoplasms; Head and Neck Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Pancreatic Neoplasms; Colonic Neoplasms; Papillomavirus Infections; Rectal Neoplasms; Carcinoma; Vaginal Neoplasms; Anus Neoplasms; Intestinal Neoplasms | Details |
| XTX-301 | XTX-301 | Phase 2 Clinical | Solid tumours; Neoplasms | Details | |
| STX-001 | STX-001 | Phase 2 Clinical | Massachusetts Institute Of Technology | Solid tumours; Triple Negative Breast Neoplasms; Melanoma | Details |
| JCXH-211 | JCXH-211 | Phase 2 Clinical | Immorna (Hangzhou) Biotechnology Co., Ltd | Solid tumours; Neoplasms; Skin Neoplasms; Glioma | Details |
| Recombinant Human nsIL12 Oncolytic Adenovirus (Beijing Haute Biotechnology) | BioTTT-001; BioTTT001 | Phase 2 Clinical | Beijing Bio-Targeting Therapeutics Technology Co Ltd | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Glioma | Details |
| Recombinant Human IL12/15-PDL1B Herpes Simplex Type I Oncolytic Virus (Cnbg-Virogin Biotech (Shanghai) Ltd) | VG-161 | Phase 2 Clinical | Cnbg-Virogin Biotech (Shanghai) Ltd | Solid tumours; Biliary Tract Neoplasms; Liver Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Sarcoma; Carcinoma, Hepatocellular | Details |
| Rocakinogene sifuplasmid | INO-9012 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Glioblastoma; Precancerous Conditions; Carcinoma, Hepatocellular; Vulvar Diseases; Lung Neoplasms; Hepatitis C; Squamous Intraepithelial Lesions of the Cervix; Colorectal Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Hemorrhagic Fever, Ebola; Urogenital Abnormalities; Papillomavirus Infections; Neoplasms, Glandular and Epithelial; Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Head and Neck Neoplasms; Ovarian Neoplasms; Hepatitis, Chronic; HIV Infections | Details |
| SFA-002 | SFA-002; SFA002 | Phase 1 Clinical | Arthritis, Rheumatoid; Plaque psoriasis | Details | |
| VG-201 | VG-201 | Phase 1 Clinical | ViroGin Biotech Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| VG-203 | VG-203; VG2062; VG-2062 | Phase 1 Clinical | ViroGin Biotech Ltd, Shanghai Funuojian Biotechnology Co Ltd, Virogin Biotech (Shenzhen) Ltd, Fenojian Biotechnology (Nantong) Co Ltd | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Breast Neoplasms | Details |
| MVR-C5252 | C-5252; MVR-C5252; T-5 | Phase 1 Clinical | Immvira Co Ltd | Solid tumours; Ganglioglioma; Glioblastoma; Brain Neoplasms; Glioma | Details |
| WTX-330 | WTX-330 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasms; Lymphoma, Non-Hodgkin | Details |
| WTX-124 | WTX-124 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours | Details |
| Ustekinumab biosimilar (Curateq Biologics) | BP38; BP-38 | Phase 1 Clinical | CuraTeQ Biologics Pvt Ltd | Psoriasis | Details |
| IL-12 DNA(NIAID) | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details | |
| SW-0715 | SW-0715 | Phase 1 Clinical | Stemirna Therapeutics | Solid tumours | Details |
| KGX-101 | KGX101; KGX-101(IL-12-Fc融合蛋白); KGX-101(改造优化的细胞因子IL12前药分子); KGX-101 | Phase 1 Clinical | Shanghai KangaBio Co Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| ABOD-2011 | ABOD-2011 | Phase 1 Clinical | Cancer Hospital Of Chinese Academy Of Medical Sciences | Solid tumours | Details |
| DF-6002 | DF-6002 | Phase 1 Clinical | Dragonfly Therapeutics Llc | Solid tumours | Details |
| EGFR IL12 CART | Phase 1 Clinical | Colorectal Neoplasms | Details | ||
| MEDI-1191 | MEDI-1191; MEDI1191 | Phase 1 Clinical | Moderna Inc | Solid tumours; Neoplasms | Details |
| MEDI-9253 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours | Details | |
| Veledimex | INXN-1001; AD-1001 | Ziopharm Oncology Inc | Details | ||
| bacTRL-IL-12 | IQVIA RDS Pty Ltd | Details | |||
| Ustekinumab biosimilar (BioFactura) | BFI-751 | BioFactura Australia Pty Ltd | Details | ||
| INXN-2001 | Ad-IL-12; ZIN-ATI-001; Ad-RTS-hIL-12; Ad-RTS-mIL-12; INXN-2001; Ad-RTS-IL-12; INXN-2001 (Ad-RTS-IL-12) | Ziopharm Oncology Inc | Details |
This web search service is supported by Google Inc.







