
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































Designed under ISO 9001:2015 and ISO 13485:2016
Manufactured and QC tested under a GMP compliance factory
Animal-Free materials
Beta-lactam materials free
Batch-to-batch consistency
Stringent quality control tests
This protein carries no "tag".
The protein has a calculated MW of 58.9 kDa. The protein migrates as 76 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
This product is supplied and shipped with blue ice, please inquire the shipping cost.
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
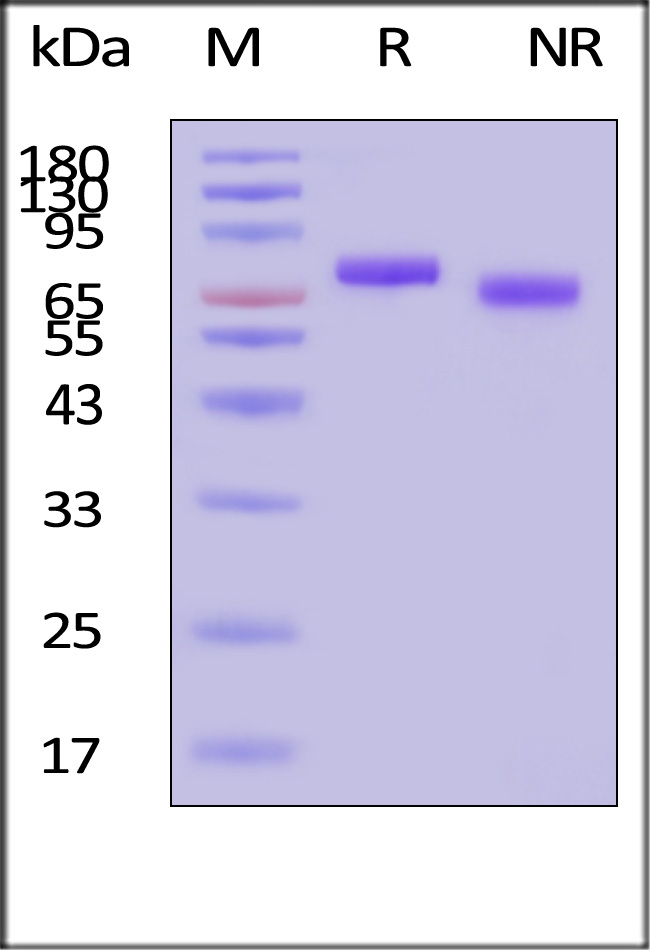
GMP Human IL-12 Protein on SDS-PAGE under reducing (R) and non-reducing (NR) conditions. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).

GMP Human IL-12 Protein (Cat. No. GMP-L12H23) stimulates secretion of IFN-γ by NK-92. The specific activity of GMP Human IL-12 Protein is > 1.00ⅹ10^7 IU/mg, which is calibrated against human IL-12 WHO International Standard (NIBSC code: 95/544) (QC tested).

The activity of GMP Human IL-12 Protein (Cat. No. GMP-L12H23) was higher than other competing products.

Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14), GMP Human IL-12 Protein (ACROBiosystems, Cat. No. GMP-L12H23) and GMP Human 4-1BB Ligand Protein (ACROBiosystems, Cat. No. GMP-41LH26), in CelThrea™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101 & GMP-CM3101-1) for two weeks. The result shows that GMP Human IL-12 Protein (ACROBiosystems) can promote the expansion of these cells with a reasonable cell viability, and can be comparable with the Competitor M IL-12 protein.

Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14), GMP Human IL-12 Protein (ACROBiosystems, Cat. No. GMP-L12H23) and GMP Human 4-1BB Ligand Protein (ACROBiosystems, Cat. No. GMP-41LH26), in CelThrea™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101 & GMP-CM3101-1) for two weeks. The result shows that GMP Human IL-12 Protein (ACROBiosystems) can be comparable with the Competitor M IL-12 protein.

The Cell based assay shows that GMP Human IL-12 Protein (Cat. No. GMP-L12H23) is stable at 37°C for 24 hours.

The Cell based assay shows that GMP Human IL-12 Protein (Cat. No. GMP-L12H23) is stable at 4℃ for 180 days.

The Cell based assay shows that GMP Human IL-12 Protein (Cat. No. GMP-L12H23) is stable after freezing and thawing 3 times.

The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG IL-12.
ACROBiosystems GMP grade products are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP<92>Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP<1043>Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems Quality Management System Contents:
Designed under ISO 9001:2015 and ISO 13485:2016, Manufactured and QC tested under a GMP compliance factory
Animal-Free materials
Materials purchased from the approved suppliers by QA
ISO 5 clean rooms and automatic filling equipment
Qualified personnel
Quality-related documents review and approve by QA
Fully batch production and control records
Equipment maintenance and calibration
Validation of analytical procedures
Stability studies conducted
Comprehensive regulatory support files
Request For Regulatory Support Files(RSF)
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
SDS-PAGE
Protein content
Endotoxin level
Residual Host Cell DNA content
Residual Host Cell Protein content
Biological activity analysis
Microbial testing
Mycoplasma testing
In vitro virus assay
Batch-to-batch consistency
Price(USD) : $1055.00
Price(USD) : $5520.00
Price(USD) : $7775.00

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides comprehensive tools for neuroscience research, including neuroscience proteins, pre-formed fibrils (PFFs), neural antibodies, neural factors, and more—aiming to accelerate disease modeling, drug screening, and neural mechanism studies.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ustekinumab | C-340; CNTO-1275 | Approved | Johnson & Johnson Innovative Medicine | Stelara, 喜达诺 | United States | Plaque psoriasis | Janssen Biotech Inc | 2009-09-25 | Multiple Sclerosis; Plaque psoriasis; Dermatitis, Atopic; Crohn Disease; Hidradenitis Suppurativa; Common Variable Immunodeficiency; Uveitis; Colitis, Ulcerative; Arthritis, Psoriatic; Takayasu Arteritis; Lupus Erythematosus, Systemic; Psoriasis; Diabetes Mellitus, Type 1; Arthritis, Juvenile; Sjogren's Syndrome; Spondylitis, Ankylosing; Pouchitis; Ichthyosis; Dermatomyositis; Inflammatory Bowel Diseases; Type I Leukocyte Adhesion Defect; Polymyositis; Liver Cirrhosis, Biliary | Details |
| Ustekinumab biosimilar(Formycon) | FYB-202 | Approved | Formycon AG | OTULFI | EU | Crohn Disease; Colitis, Ulcerative | Fresenius Kabi Deutschland Gmbh, Formycon AG | 2024-09-25 | Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab Biosimilar (qyuns) | QX-001S; QX-001-S; HDM-3001 | Approved | Qyuns Therapeutics Co Ltd | Mainland China | Plaque psoriasis | Qyuns Therapeutics Co Ltd | 2024-10-29 | Psoriasis; Plaque psoriasis | Details | |
| Ustekinumab Biosimilar(Celltrion) | CT-P43 | Approved | Celltrion Inc | Steqeyma, Steqeyma IV | Canada | Plaque psoriasis; Arthritis, Psoriatic; Crohn Disease | Celltrion Inc | 2024-08-01 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar (Biocon) | Approved | Biocon Biologics Ltd | Bmab 1200, Yesintek | United States | Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease | Biocon Biologics Inc | 2024-11-29 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details | |
| Ustekinumab biosimilar (Alvotech) | AVT04; AVT-04 | Approved | Alvotech hf | Jamteki, Uzpruvo, SELARSDI™, JamtekiTM, Ustekinumab BS (F) | Japan | Arthritis, Psoriatic; Psoriasis | Fuji Pharma Co Ltd | 2023-09-25 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Plaque psoriasis; Crohn Disease | Details |
| Ustekinumab biosimilar(Amgen) | ABP-654 | Approved | Amgen Inc | Wezlana, WEZLANA, wezenla | United States | Crohn Disease; Colitis, Ulcerative; Arthritis, Psoriatic; Plaque psoriasis | Amgen Inc | 2023-10-31 | Psoriasis; Arthritis, Psoriatic; Colitis, Ulcerative; Crohn Disease; Plaque psoriasis | Details |
| Thalidomide | NSC-66847; NSC-527179; K-17; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | Xerostomia; Colorectal Neoplasms; Neuroectodermal Tumors, Primitive; Prostatitis; Primary Myelofibrosis; Osteosarcoma; Cholangitis, Sclerosing; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Adenocarcinoma, Clear Cell; Carcinoma, Adenosquamous; Epilepsy; Erythema Nodosum; Sarcoma; HIV Wasting Syndrome; Pancreatitis, Chronic; Sarcoma, Ewing; Arachnoiditis; Gastrointestinal Hemorrhage; Retinoblastoma; Prostatic Neoplasms; Endometriosis; Lung Neoplasms; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Myelodysplastic-Myeloproliferative Diseases; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Carcinoma, Hepatocellular; Burning Mouth Syndrome; Stomatitis; Neoplasm Metastasis; Appendiceal Neoplasms; Tuberculosis; Lymphoma, Non-Hodgkin; Angiodysplasia; Pelvic Pain; Glioma; Waldenstrom Macroglobulinemia; Leprosy, Lepromatous; Endometrial Neoplasms; Uterine Neoplasms; Anemia, Sideroblastic; Ovarian Neoplasms; Gastric Antral Vascular Ectasia; Lupus Erythematosus, Discoid; Carc | Details |
| Ustekinumab biosimilar(DM Bio) | DA-3115; DMB-3115 | Approved | Dong-A Pharmaceutical Co Ltd, Meiji Seika Pharma Co Ltd | IMULDOSA, Absimky | United States | Psoriasis | Dong-A ST Co Ltd, Meiji Seika Kaisha Ltd | 2024-10-11 | Psoriasis; Colitis, Ulcerative; Crohn Disease | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Lisofylline | BL-194; CT-1501R; A-802710 | Phase 3 Clinical | Cti Biopharma Corp | Diabetes Mellitus, Type 1; Rejection of organ transplantation | Details |
| Apilimod mesilate | AIT-101; STA-5326-mesylate; LAM-002; STA-5326; LAM-002A | Phase 2 Clinical | Madrigal Pharmaceuticals Inc, Lam | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Common Variable Immunodeficiency; Crohn Disease; Amyotrophic Lateral Sclerosis | Details |
| NSC-733972 | M-032; M032-H SV-1; NSC-733972 | Phase 2 Clinical | University Of Alabama At Birmingham | Glioblastoma; Gliosarcoma; Astrocytoma | Details |
| Tavokinogene telseplasmid | DNA IL-12; pIL-12 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | HIV Infections; Hemorrhagic Fever, Ebola; Carcinoma, Merkel Cell; Squamous Cell Carcinoma of Head and Neck; Triple Negative Breast Neoplasms; Neoplasms; Prostatic Neoplasms; Hepatitis B; Lymphoma, T-Cell, Cutaneous; Melanoma; Mycosis Fungoides | Details |
| T-3011 | T-3 (ImmVira Pharma); MVR-T3011; T3011; MVR-T3011 IT; MVR-T3011 IV; B015; B-015 | Phase 2 Clinical | Immvira Co Ltd | Sarcoma; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular; Melanoma; Endometrial Neoplasms; Lung Neoplasms; Lymphoma; Carcinoma, Squamous Cell; Colorectal Neoplasms; Breast Neoplasms; Solid tumours; Urinary Bladder Neoplasms; Mesothelioma; Ascites; Skin Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Ovarian Neoplasms; Liver Neoplasms; Head and Neck Neoplasms | Details |
| PDS-0301 | M-9241; MSB-0010360; MSB0010360; PDS-0301 | Phase 2 Clinical | Emd Serono Inc, Merck Serono, National Cancer Institute | Prostatic Neoplasms; Sarcoma, Kaposi; Uterine Cervical Neoplasms; Urogenital Neoplasms; Metastatic breast cancer; Penile Neoplasms; Bile Duct Neoplasms; Colorectal Neoplasms; Oropharyngeal Neoplasms; Cholangiocarcinoma; Vulvar Neoplasms; Breast Neoplasms; Head and Neck Neoplasms; Urinary Bladder Neoplasms; Prostatic Neoplasms, Castration-Resistant; Pancreatic Neoplasms; Colonic Neoplasms; Papillomavirus Infections; Rectal Neoplasms; Carcinoma; Vaginal Neoplasms; Anus Neoplasms; Intestinal Neoplasms | Details |
| XTX-301 | XTX-301 | Phase 2 Clinical | Solid tumours; Neoplasms | Details | |
| STX-001 | STX-001 | Phase 2 Clinical | Massachusetts Institute Of Technology | Solid tumours; Triple Negative Breast Neoplasms; Melanoma | Details |
| JCXH-211 | JCXH-211 | Phase 2 Clinical | Immorna (Hangzhou) Biotechnology Co., Ltd | Solid tumours; Neoplasms; Skin Neoplasms; Glioma | Details |
| Recombinant Human nsIL12 Oncolytic Adenovirus (Beijing Haute Biotechnology) | BioTTT-001; BioTTT001 | Phase 2 Clinical | Beijing Bio-Targeting Therapeutics Technology Co Ltd | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Glioma | Details |
| Recombinant Human IL12/15-PDL1B Herpes Simplex Type I Oncolytic Virus (Cnbg-Virogin Biotech (Shanghai) Ltd) | VG-161 | Phase 2 Clinical | Cnbg-Virogin Biotech (Shanghai) Ltd | Solid tumours; Biliary Tract Neoplasms; Liver Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Cholangiocarcinoma; Sarcoma; Carcinoma, Hepatocellular | Details |
| Rocakinogene sifuplasmid | INO-9012 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Glioblastoma; Precancerous Conditions; Carcinoma, Hepatocellular; Vulvar Diseases; Lung Neoplasms; Hepatitis C; Squamous Intraepithelial Lesions of the Cervix; Colorectal Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Hemorrhagic Fever, Ebola; Urogenital Abnormalities; Papillomavirus Infections; Neoplasms, Glandular and Epithelial; Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Head and Neck Neoplasms; Ovarian Neoplasms; Hepatitis, Chronic; HIV Infections | Details |
| SFA-002 | SFA-002; SFA002 | Phase 1 Clinical | Arthritis, Rheumatoid; Plaque psoriasis | Details | |
| VG-201 | VG-201 | Phase 1 Clinical | ViroGin Biotech Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| VG-203 | VG-203; VG2062; VG-2062 | Phase 1 Clinical | ViroGin Biotech Ltd, Shanghai Funuojian Biotechnology Co Ltd, Virogin Biotech (Shenzhen) Ltd, Fenojian Biotechnology (Nantong) Co Ltd | Solid tumours; Ovarian Neoplasms; Carcinoma, Renal Cell; Breast Neoplasms | Details |
| MVR-C5252 | C-5252; MVR-C5252; T-5 | Phase 1 Clinical | Immvira Co Ltd | Solid tumours; Ganglioglioma; Glioblastoma; Brain Neoplasms; Glioma | Details |
| WTX-330 | WTX-330 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours; Neoplasms; Lymphoma, Non-Hodgkin | Details |
| WTX-124 | WTX-124 | Phase 1 Clinical | Werewolf Therapeutics Inc | Solid tumours | Details |
| Ustekinumab biosimilar (Curateq Biologics) | BP38; BP-38 | Phase 1 Clinical | CuraTeQ Biologics Pvt Ltd | Psoriasis | Details |
| IL-12 DNA(NIAID) | Phase 1 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid) | HIV Infections | Details | |
| SW-0715 | SW-0715 | Phase 1 Clinical | Stemirna Therapeutics | Solid tumours | Details |
| KGX-101 | KGX101; KGX-101(IL-12-Fc融合蛋白); KGX-101(改造优化的细胞因子IL12前药分子); KGX-101 | Phase 1 Clinical | Shanghai KangaBio Co Ltd | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| Ustekinumab Biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Autoimmune Diseases | Details | |
| TG-6050 | TG-6050 | Phase 1 Clinical | Transgene Sa | Carcinoma, Non-Small-Cell Lung | Details |
| Ustekinumab biosimilar(Rani Therapeutics) | RT-111 | Phase 1 Clinical | Rani Therapeutics Llc | Psoriasis | Details |
| ABOD-2011 | ABOD-2011 | Phase 1 Clinical | Cancer Hospital Of Chinese Academy Of Medical Sciences | Solid tumours | Details |
| DF-6002 | DF-6002 | Phase 1 Clinical | Dragonfly Therapeutics Llc | Solid tumours | Details |
| EGFR IL12 CART | Phase 1 Clinical | Colorectal Neoplasms | Details | ||
| MEDI-1191 | MEDI-1191; MEDI1191 | Phase 1 Clinical | Moderna Inc | Solid tumours; Neoplasms | Details |
| MEDI-9253 | Phase 1 Clinical | Astrazeneca Plc | Solid tumours | Details | |
| Veledimex | INXN-1001; AD-1001 | Ziopharm Oncology Inc | Details | ||
| bacTRL-IL-12 | IQVIA RDS Pty Ltd | Details | |||
| Ustekinumab biosimilar (BioFactura) | BFI-751 | BioFactura Australia Pty Ltd | Details | ||
| INXN-2001 | Ad-IL-12; ZIN-ATI-001; Ad-RTS-hIL-12; Ad-RTS-mIL-12; INXN-2001; Ad-RTS-IL-12; INXN-2001 (Ad-RTS-IL-12) | Ziopharm Oncology Inc | Details |
This web search service is supported by Google Inc.







