
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>




































GMP Human IL-4 Protein (Cat. No. GMP-L04H26) stimulates proliferation of TF-1 human erythroleukemic cell line. The specific activity of GMP Human IL-4 Protein is > 1.20×10^7 IU/mg, which is calibrated against human IL-4 WHO International Standard (NIBSC code: 88/656) (QC tested).
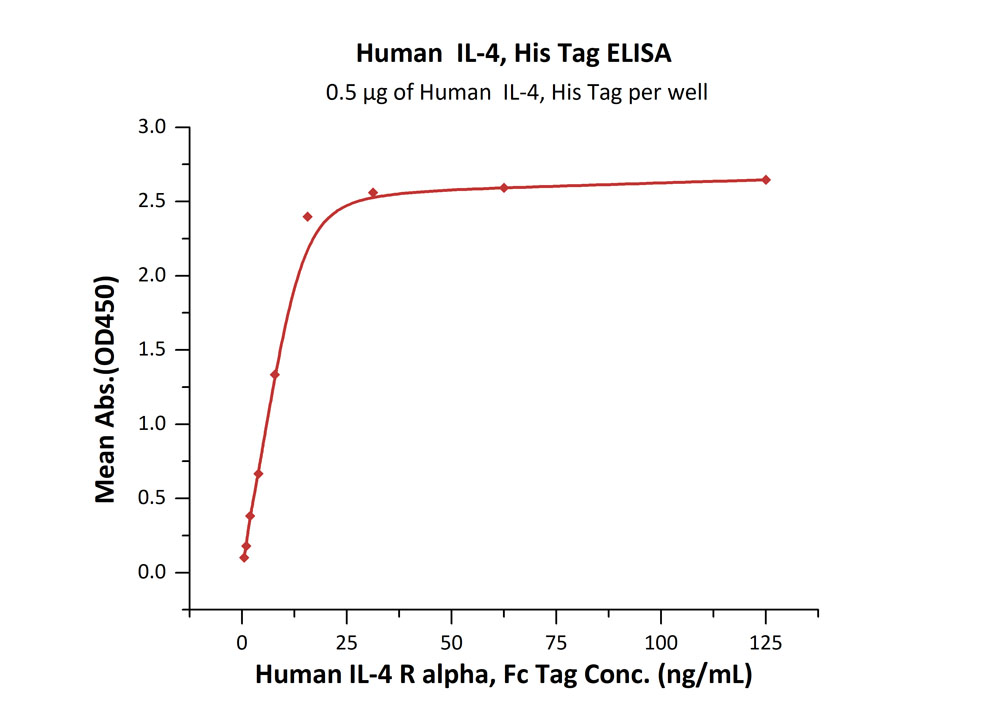
Immobilized Human IL-4, His Tag (Cat. No. IL4-H52H9) at 5 μg/mL (100 μL/well) can bind Human IL-4 R alpha, Fc Tag (Cat. No. ILR-H5253) with a linear range of 0.5-16 ng/mL (QC tested).
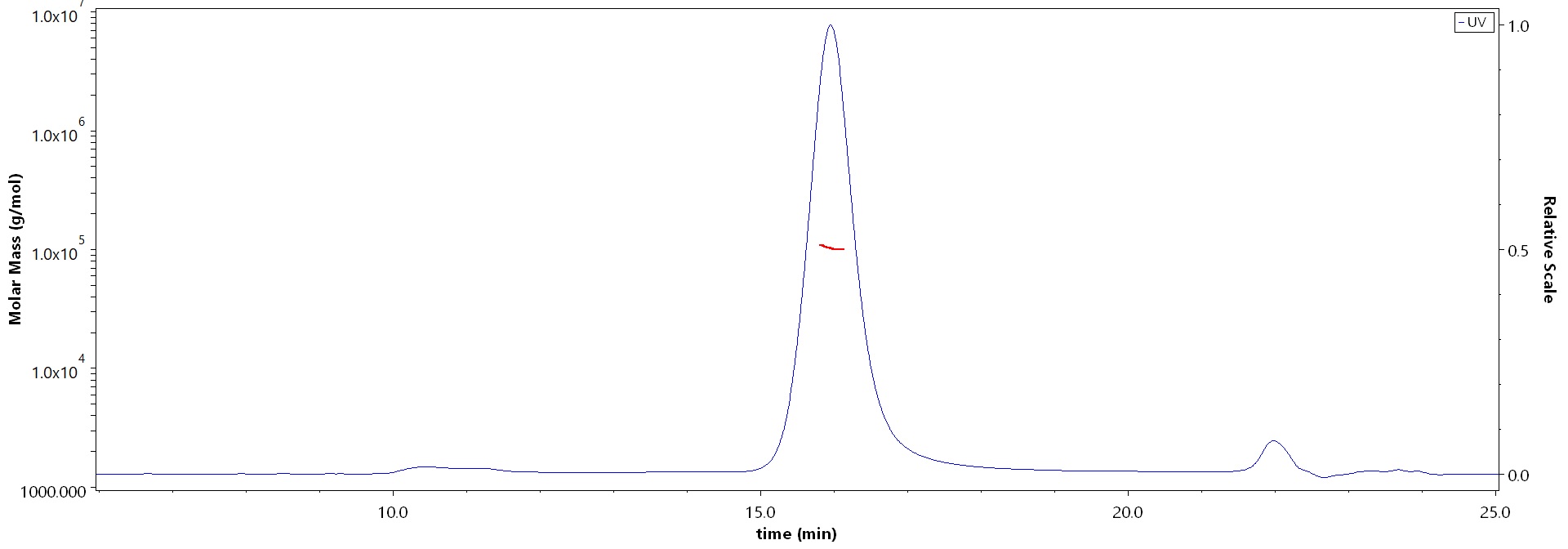
The purity of Human IL-4 Protein, Fc Tag (Cat. No. IL4-H5253) is more than 90% and the molecular weight of this protein is around 90-110 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Dupilumab | SAR-231893; REGN-668 | Approved | Sanofi, Regeneron Pharmaceuticals Inc | Dupixent, 达必妥 | United States | Dermatitis, Atopic | Regeneron Pharmaceuticals Inc | 2017-03-28 | Scleroderma, Localized; Sleep Apnea Syndromes; Sinusitis; Duodenitis; Urticaria; Colitis, Ulcerative; Asthma; Pemphigoid, Bullous; Prurigo; Neurodermatitis; Paranasal Sinus Diseases; Conjunctivitis, Allergic; Pulmonary Disease, Chronic Obstructive; Asthma, Aspirin-Induced; Peanut Hypersensitivity; Hypersensitivity, Immediate; Keratoconjunctivitis; Keloid; Dermatitis, Atopic; Eczema; Hypersensitivity; Angioedema; Milk Hypersensitivity; Aspergillosis, Allergic Bronchopulmonary; Lichenoid Eruptions; Hypereosinophilic Syndrome; Gastrointestinal Diseases; Pruritus; Respiratory Sounds; Alopecia Areata; Chronic Urticaria; Respiration Disorders; Nasal Polyps; Eosinophilic gastroenteritis (EG); Respiratory Tract Diseases; Coronavirus Disease 2019 (COVID-19); Prurigo Nodularis; Dermatitis; Skin Diseases, Eczematous; Genetic Diseases, Inborn; Skin Diseases; Prostatic Neoplasms; Eosinophilic Esophagitis; Rhinitis, Allergic | Details |
| Berdazimer sodium | NVN-1000-SB-206-Novan; SB-206; MAP3-NONOate - SB-206; NVN1000-SB206; SB-019; NI-MC101; SB-207; SB-208; SB-204; SB-414 | Approved | Novan Inc | Zelsuvmi, ZELSUVMI, KINSOLUSTM | United States | Molluscum Contagiosum | Lnhc Inc | 2024-01-05 | Acne Vulgaris; Tinea Pedis; Psoriasis; Molluscum Contagiosum; Condylomata Acuminata; Dermatitis, Atopic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Eblasakimab | MK-6105; ASLAN-004; CSL-334 | Phase 2 Clinical | Csl Ltd, Merck Sharp & Dohme Corp | Dermatitis, Atopic; Hypersensitivity | Details |
| Dectrekumab/VAK-694 | QBX-258 | Phase 2 Clinical | Novartis Pharma Ag | Lymphedema; Asthma | Details |
| OCH-NCNP1 | OCH-NCNP1 | Phase 2 Clinical | Keio University | Multiple Sclerosis; Crohn Disease | Details |
| PF-07264660 | PF-07264660 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| PF-07275315 | PF-07275315 | Phase 2 Clinical | Pfizer Inc | Dermatitis, Atopic | Details |
| AUP1602-C | AUP-16; AUP1602-C | Phase 2 Clinical | Aurealis | Diabetic Foot; Ulcer | Details |
| KBL-693 | KBL-693 | Phase 1 Clinical | Kobiolabs | Asthma | Details |
This web search service is supported by Google Inc.







